the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mckelveyite group minerals – Part 3: Bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O, a new species from Mont Saint-Hilaire, Canada
Inna Lykova
Ralph Rowe
Glenn Poirier
Henrik Friis
Kate Helwig
The new mckelveyite group mineral bainbridgeite-(YCe), ideally Na2Ba2YCe(CO3)6 ⋅ 3H2O, was found at Mont Saint-Hilaire, Quebec, Canada. Bainbridgeite-(YCe) occurs as pseudotrigonal and pseudohexagonal hemimorphic crystals that show platy, columnar, tabular, cone-shaped, barrel-shaped, saucer-shaped, or spindle-shaped habit. They often form stacked or parallel growth aggregates, rosettes, and groups of radiating crystals. The crystals are usually less than 1 mm in size. Bainbridgeite-(YCe) varies in colour from pale yellow to yellow, grey to almost black, bluish grey, green-grey, or white. The streak is white; the lustre is vitreous. The mineral has no cleavage. The Mohs hardness is 3. Dcalc is 3.49 g cm−3. Bainbridgeite-(YCe) is optically biaxial (+), α= 1.572(2), β= 1.586(2), γ= 1.628(2), 2 V (calc.) = 62∘, 2 V (meas.) = 45(4)∘(589 nm). The IR spectrum is reported. The composition (wt %, average of five analyses) is Na2O 6.86, CaO 0.59, SrO 4.01, BaO 25.71, Y2O3 8.24, La2O3 4.96, Ce2O3 8.38, Pr2O3 0.48, Nd2O3 1.87, Sm2O3 0.23, Gd2O3 0.67, Tb2O3 0.07, Dy2O3 1.38, Ho2O3 0.32, Er2O3 0.94, Tm2O3 0.08, Yb2O3 0.49, CO2 27.03, H2O 5.67, total 97.98. The empirical formula of the holotype calculated on the basis of six cations is as follows: Na2.11Ca0.10Sr0.37Ba1.60Y0.70La0.29Ce0.49Pr0.03Nd0.11Sm0.01Gd0.03Dy0.07Ho0.02Er0.05 Yb0.02(CO3)5.86(H2O)3.00. The mineral is triclinic, P1, a= 9.1079(2) Å, b= 9.1066(3) Å, c= 6.9332(2) Å, α= 102.861(2)∘, β= 116.148(2)∘, γ= 60.181(2)∘, V= 447.85(2) Å3, and Z= 1. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are 6.22(42)(001, 1, 01), 4.430(100)(01, 1, 120), 4.094(37)(1, 11, 210, 111), 3.263(26)(11, 2, 121), 2.888(67)(2, 12, 211), 2.633(38)(01, 030, 1), 2.263(23)(21, 1, 1). 2.010(20)(02, 3, 03, 301, 032, 331). The crystal structure, solved and refined from single-crystal X-ray diffraction data (R1= 0.040), is of the weloganite type.
- Article
(9450 KB) - Full-text XML
- Companion paper 1
- Companion paper 2
- Companion paper 4
-
Supplement
(466 KB) - BibTeX
- EndNote
This paper describes bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O, a new member of the mckelveyite group. The group includes rare carbonates with the general formula A3B3(CO3)6 ⋅ 3H2O, where A= Na, Ca, Y, and Zr, and B= Sr, Ba, Ce, and La (Lykova et al., 2023a). Six other members are known: mckelveyite-(Y), NaCaBa3Y(CO3)6 ⋅ 3H2O) (Milton et al., 1965), donnayite-(Y) NaCaSr3Y(CO3)6 ⋅ 3H2O (Chao et al., 1978), ewaldite BaCa(CO3)2 ⋅ 2.6H2O (Donnay et al., 1971), weloganite Na2Sr3Zr(CO3)6 ⋅ 3H2O (Sabina et al., 1968), alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O (Lykova et al., 2023b), and alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O (IMA 2021-047).
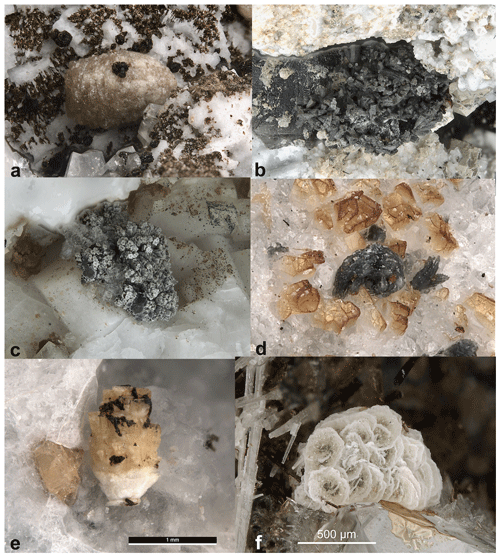
Figure 1Bainbridgeite-(YCe) from Mont Saint-Hilaire, Quebec, Canada: (a) pale-orange-yellow barrel-shaped prismatic crystal 2.5 mm in size with brown stilpnomelane. Specimen CMNMC 46324 (the holotype); (b) a vug encrusted with dark-grey elongated cone-shaped crystals. FOV 7 mm. Specimen CMNMC 89599; (c) a cluster of grey columnar crystals 2.5 mm in size. Specimen CMNMC 89601; (d) grey rosette 0.3 mm in size. Specimen CMNMC 89613; (e) yellow stacked 1.3 mm crystal. CMNMC 87937; (f) 1 mm aggregated of stacked zoned platy crystals with grey-green cores and white rims. CMNMC 91452. Canadian Museum of Nature collection. Photos: François Génier (a–d) and Michael Bainbridge (e–f).
The mineral with chemical formula Na2Ba2YCe(CO3)6 ⋅ 3H2O was first found and marked as an unknown phase UK109 from Mont Saint-Hilaire, Quebec, Canada, by Andrew M. McDonald in 1998 (Horvath et al., 2019). The poor quality of UK109 crystals, typical for the mckelveyite group minerals, presented a major challenge to studying the phase, and thus the mineral has not been formally described until now.
Bainbridgeite-(YCe) is named in honour of Michael Bainbridge (born 1974), a Canadian mineralogist, prominent mineral collector, photographer, and writer. Michael has been an invaluable contributor to the Canadian mineralogical community and a tireless promoter of mineralogy. He is the author of the Minerals of Grenville Province and The Pinch Collection at the Canadian Museum of Nature books, as well as a major contributor to the latest monograph on the minerals of Mont Saint-Hilaire by Horvath et al. (2019). In January 2023 Michael joined the Canadian Museum of Nature collections staff.
The parenthesised Levinson suffix -(YCe) was added in accordance with the nomenclature for rare-earth and Y mineral species (Levinson, 1966; Bayliss and Levinson, 1988) and the recently approved nomenclature of the mckelveyite group; the first symbol represents the dominant cations at one of the A sites and the second symbol at one of the B sites (Lykova et al., 2023a).

Figure 2Bainbridgeite-(YCe) crystal. Darker areas correspond to an intermediate member of the bainbridgeite-(YCe)–alicewilsonite-(YCe) series; the bright rim is characterised by a lower Y and a higher Ln content. Polished section. SEM (BSE) image.
Both the new mineral and the name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA CNMNC), proposal IMA 2020-065. The holotype of bainbridgeite-(YCe) is the specimen with the catalogue number CMNMC 46324 from the collection of the Canadian Museum of Nature, Canada, originally labelled as “mckelveyite-(Y)”.
Bainbridgeite-(YCe) is a late-stage mineral found in hydrothermally altered alkaline pegmatites, miarolitic cavities in nepheline and sodalite syenite, and marble xenoliths at the Poudrette (Demix) quarry, Mont Saint-Hilaire, Quebec, Canada (Horvath et al., 2019, and the references therein). It is the second most common mckelveyite group mineral at the locality after alicewilsonite-(YCe). Bainbridgeite-(YCe) commonly occurs with calcite, quartz, microcline, pectolite, dolomite, siderite, zeolites (natrolite, phillipsite, analcime, gmelinite), aegirine, elpidite, ilmenite, gaidonnayite, hilairite, lorenzenite, brookite, and bastnaesite group minerals. It is not unusual to find the latter in close proximity to bainbridgeite-(YCe) and sometimes intergrown with one another.
Bainbridgeite-(YCe) crystals are hemimorphic pseudotrigonal and pseudohexagonal and show a wide range of habits, including platy, columnar, tabular, cone-shaped, barrel-shaped, saucer-shaped, and spindle-shaped (Fig. 1). The crystal faces are often curved, split, and/or stepped. They commonly form stacked or parallel growth aggregates, rosettes, and groups of radiating crystals. The crystals are usually less than 1 mm in size with some reaching 1.5–2 mm. Aggregates can reach 3 mm in size. Generally, bainbridgeite-(YCe) is indistinguishable in hand specimens from other mckelveyite group minerals, although dark-grey to almost-black mckelveyite-like phases are likely to correspond to bainbridgeite-(YCe) (Fig. 1b–d).
Bainbridgeite-(YCe) and alicewilsonite-(YCe) are sometimes found forming epitactic overgrowths on each other. Mckelveyite-(Y) and donnayite-(Y) also form epitactic relationships with bainbridgeite-(YCe).
The bainbridgeite-(YCe) type material was found by Elsa Pfenninger-Horvath and Laszlo Horvath on 25 and 26 February 1978 (Laszlo Horvath, personal communication, 2020). At the time there were two independently operating quarries, separated by a dividing wall: Demix and Poudrette. The material was collected in the former Demix quarry, which is now the western half of the unified quarries. Bainbridgeite-(YCe) was found in a heavily altered body mostly consisting of calcite and albite in nepheline syenite. This geological environment can be classified as a “carbonate pegmatite” (Normand and Tarassoff, 2006), also known as a “carbonate vug” (Chao et al., 1967) based on the absence of minerals essential in the alkaline pegmatites and the lack of pyroxene and amphibole group minerals, as well as the abundance of calcite. No systematic study has ever been carried out on “carbonate pegmatites”, and little is known about their origin (Normand and Tarassoff, 2006; Horvath et al., 2019). Bainbridgeite-(YCe) forms barrel-shaped short prismatic crystals up to 2 mm in length (Figs. 1a, 2). The crystals are found sparsely distributed on albite encrusted with scaly brown stilpnomelane.
Bainbridgeite-(YCe) varies in colour from pale yellow to yellow, orange-yellow, orange, grey to almost black, bluish grey, green-grey, green, or even white (Fig. 1). Grey and black colours are most common. The streak is white; the lustre is vitreous. The mineral has no cleavage, and its fracture is uneven. The Mohs hardness is 3. The mineral is non-fluorescent under ultraviolet light. The density calculated using the empirical formula and unit-cell volume refined from the single-crystal XRD data is 3.49 g cm−3.
Bainbridgeite-(YCe) is optically biaxial (+), α= 1.572(2), β= 1.586(2), γ= 1.628(2), 2 V (meas.) = 48(4)∘ (from a spindle-stage extinction curve), 2 V (calc.) = 62∘.
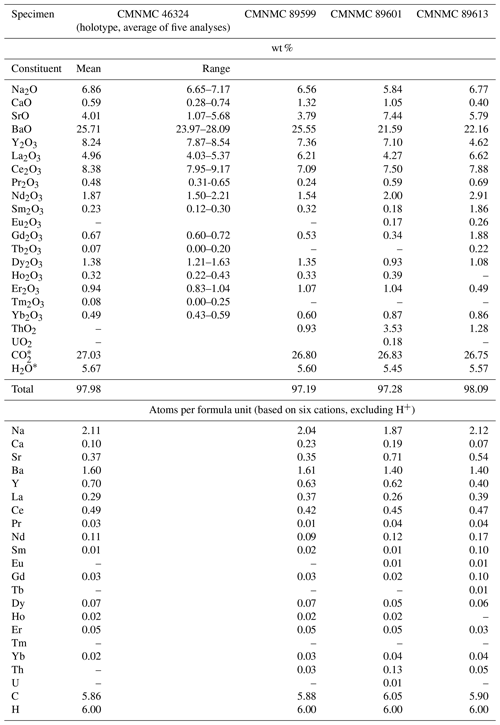
Electron microprobe analyses (EMPAs) for bainbridgeite-(YCe) were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five WDS spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) using an acceleration voltage of 20 kV, a beam current of 10 nA, and a beam diameter of 20–50 µm depending on the grain size. Bainbridgeite-(YCe) is unstable under an electron beam, and so a larger beam diameter was used to minimise element migration. The following reference materials were used: albite or NaInSi2O6 (NaKα), diopside (CaKα), celestine (SrLα), sanbornite (BaLα), YAG (YLα), LaPO4 (LaLα), CePO4 (CeLα), PrPO4 (PrLβ), NdPO4 (NdLα), SmPO4 (SmLα), EuPO4 (EuLα), GdPO4 (GdLα), TbPO4 (TbLα), DyPO4 (DyLβ), HoPO4 (HoLβ), ErPO4 (ErLα), TmPO4 (TmLα), YbPO4 (YbLα), ThO2 (ThMα), and UO2 (UMα). The intensity data were corrected for time-dependent intensity (TDI) loss (or gain) using a self-calibrated correction for NaKα, CaKα, YLα, and LaLα. H2O and CO2 contents were not analysed due to the paucity of the available material.
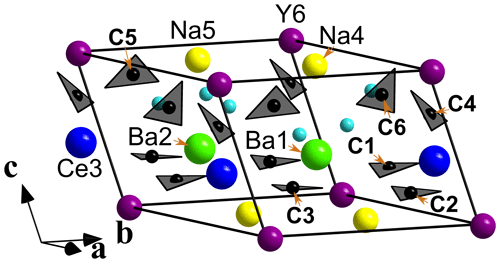
Figure 4General view of the crystal structure of bainbridgeite-(YCe). Sky-blue spheres are H2O molecules. The unit cell is outlined.
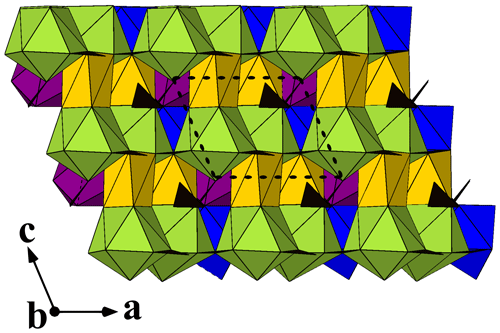
Figure 5A view along [010] of the crystal structure of bainbridgeite-(YCe). Ba-, Ce-, Y-, and Na-centred polyhedra are shown in green, blue, purple, and yellow, respectively. Carbonate groups are black triangles. The unit cell is outlined.
The Fourier transform infrared (FTIR) spectrum of bainbridgeite-(YCe) was obtained using a Bruker Hyperion 2000 microscope interfaced to a Tensor 27 spectrometer with a wide-band mercury cadmium telluride (MCT) detector (Canadian Conservation Institute, Canada). A small fragment of bainbridgeite-(YCe) was mounted on a low-pressure diamond anvil microsample cell and analysed in transmission mode. The spectrum was collected between 4000–400 cm−1 with the co-addition of 150 scans at a 4 cm−1 resolution.
Powder X-ray diffraction (PXRD) data were collected at the Canadian Museum of Nature, Canada, using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and IµS microfocus X-ray source (λCuKα1=1.54060 Å) with the Kα2 contribution removed using the “Strip Kα2” tool in Bruker Diffrac.EVA V4.3. The instrument was calibrated using a statistical calibration method (Rowe, 2009). A powder ball of bainbridgeite-(YCe) ∼ 200 µm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10∘ rocking motion along the Psi axis of the Centric Eulerian Cradle stage.
Single-crystal X-ray diffraction (SXRD) studies were carried out at the Natural History Museum, University of Oslo, Norway, using a Rigaku XtaLAB Synergy-S diffractometer equipped with a HyPix 6000HE detector (λMoKα=0.71073 Å) operating at 50 kV and 1 mA. The data were processed, including absorption correction, using Rigaku's CrysAlis Pro software.
Table 2X-ray powder diffraction data (d in Å) for bainbridgeite-(YCe). The strongest reflections are given in bold.
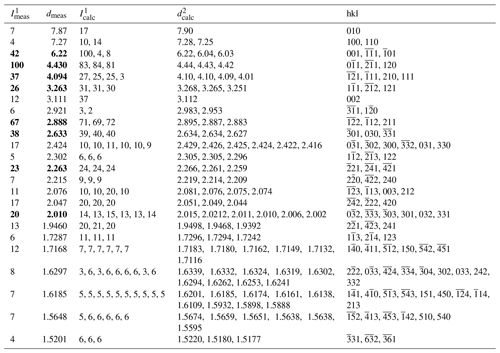
1 Calculated from the crystal structure determination; only reflections with intensities > 1 are given. 2 Calculated from PXRD Rietveld unit-cell refinement with a= 9.1079(2), b= 9.1066(3), c= 6.9332(2) Å, α= 102.861(2)∘, β= 116.148(2)∘, γ= 60.181(2)∘, and V= 447.85(2) Å3.
5.1 Chemical data
Representative EMPAs for bainbridgeite-(YCe) collected on several specimens from Mont Saint-Hilaire are given in Table 1. The contents of Zr, Lu, and Hf are below the detection limit.
The empirical formula of the holotype calculated on the basis of six cations, excluding H+, is Na2.11Ca0.10Sr0.37Ba1.60Y0.70La0.29Ce0.49Pr0.03 Nd0.11Sm0.01 Gd0.03Dy0.07Ho0.02 Er0.05Yb0.02(CO3)5.86(H2O)3.00.
The simplified formula is
Na2(Ba,Sr,Ca)2(Y,Dy,Er,Na)(Ce,La,Nd)(CO3)6 ⋅ 3H2O.
The ideal end-member formula is
Na2Ba2YCe(CO3)6 ⋅ 3H2O, which requires Na2O 6.43, BaO 31.82, Y2O3 11.71, Ce2O3 17.03, CO3 27.40, H2O 5.61, total 100 wt %.
Bainbridgeite-(YCe) dissolves in an aqueous HCl solution at room temperature with strong effervescence.
Table 3Crystal data, data collection information, and structure refinement details for bainbridgeite-(YCe).
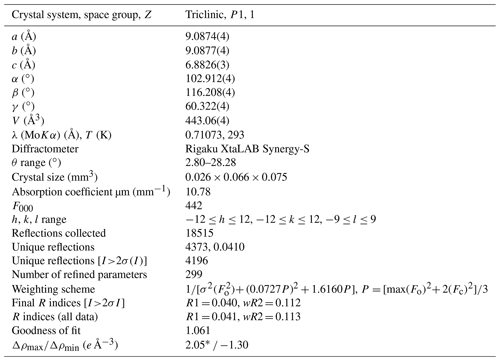
* Located 0.77 Å away from the Ce(3) site. There are several 1.7–2.1 e Å−3 peaks located 0.7–0.8 Å away from large cations sites that indicate a relatively poor overall quality of the data (caused by a poor quality of bainbridgeite-(YCe) crystals) leading to spurious peaks of residual electron density.
Table 4Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms, site occupancies, and bond-valence sums (BVSs) for bainbridgeite-(YCe).
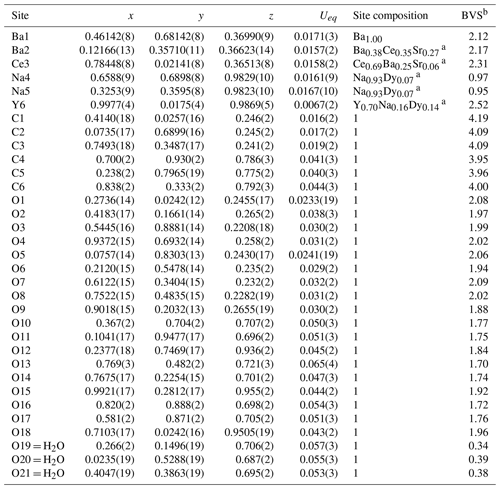
a The sites occupancies were refined assuming full occupancy. The best agreement was obtained with Ba0.763(10)Sr0.237(10) for the Ba2 site, Ce0.913(9)Sr0.087(10) for the Ce3 site; Na0.942(5)Dy0.058(5) for the Na4, Na0.928(5)Dy0.072(5) for the Na5 site, and Y0.631(15)Dy0.209(9)Na0.16 for the Y6 site. In the final refinement cycles the occupancies were fixed based on the eref values, EMPA, interatomic distances, bond-valence calculations, and the charge balance. b Bond-valence parameters were taken from Gagné and Hawthorne (2015).
5.2 Infrared spectroscopy
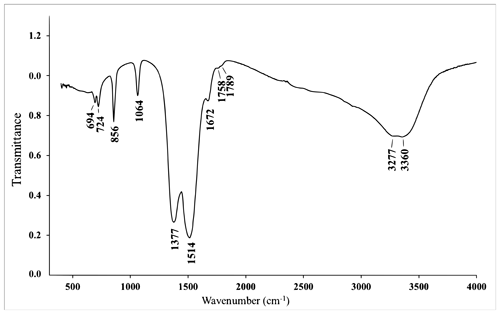
The IR spectrum of bainbridgeite-(YCe) (Fig. 3) shows IR bands of O–H stretching (in the range from 3270 to 3360 cm−1) and H–O–H bending (at 1672 cm−1) vibrations of H2O molecules and C–O stretching (at 1377 and 1514 cm−1) vibrations of CO group molecules. The band at 1064 cm−1 can be assigned to the non-degenerate mode of C–O stretching vibrations, indicating polarisation of CO groups, as this mode would have been inactive, if symmetric non-polarised carbonate groups (with a three-fold axis) were present in the IR spectrum. The band assignment was made in accordance with Chukanov and Chervonnyi (2016).
5.3 X-ray diffraction data and description of the crystal structure
The indexed PXRD data are given in Table 2. Parameters of the triclinic unit cell refined from the data are as follows: a= 9.1079(2) Å, b= 9.1066(3) Å, c= 6.9332(2) Å, α= 102.861(2)∘, β = 116.148(2)∘, γ= 60.181(2)∘, and V= 447.85(2) Å3. The PXRD pattern in the xy format is available as a file in the Supplement.
The single-crystal X-ray diffraction data were indexed in the P1 space group with the following unit-cell parameters: a= 9.0874(4) Å, b= 9.0877(4) Å, c= 6.8826(3) Å, α= 102.912(4)∘, β = 116.208(4)∘, γ= 60.322(4)∘, and V= 443.06(4) Å3. The structure was solved and refined to R1 = 0.040 on the basis of 4196 independent reflections with I>2σ(I) using the SHELXL 2018/3 program package (Sheldrick, 2015). Crystal data, data collection information, and structure refinement details are given in Table 3, atom coordinates, equivalent displacement parameters, site composition, and bond-valence sums (BVSs) in Table 4, and selected interatomic distances in Table 5. The studied crystal demonstrated twinning by merohedry Class I (Nespolo and Ferraris, 2000), with twin domains ratio of 21:79. The crystallographic information file (CIF) for bainbridgeite-(YCe) is available as a file in the Supplement. It was also deposited in the Inorganic Crystal Structure Database (ICSD; no. CSD 2297132).
SXRD studies of the mckelveyite group minerals are very challenging because of the poor quality of their crystals resulting in multiple split reflections and streaks. Bainbridgeite-(YCe) is no exception, and the structure could only be refined to R1= 0.040.
Bainbridgeite-(YCe) is strongly pseudotrigonal. There are six independent large cation sites in the structure (Fig. 4) forming two alternating layers parallel to the ab plane (Fig. 5). Na, Ba, Y, Ce, Sr, and Dy were distributed among these sites based on the EMPA, refined site-scattering factors (eref, in electrons per site), and charge balance taking into account bond-valence sums (BVSs) and interatomic distances (Tables 4–6). Ca atoms were not included in the refinement; lighter lanthanoids (Ln, La–Lu) were formally refined as Ce atoms, heavier Ln – as Dy atoms. One of the layers is formed by the Ba1, Ba2, and Ce3 sites that have 10-fold coordination. The large Ba1-centred polyhedron with the < Ba1–O > distance of 2.80 Å is occupied by Ba atoms. The refinement showed that the remaining Ba, Ce, and Sr atoms are distributed between Ba2 and Ce3 sites. Their occupancies were refined as Ba0.763(10)Sr0.237(10) (eref= 51.7) for the Ba2 site and Ce0.913(9)Sr0.087(10) (eref= 56.3) for the Ce3 site (Table 6). Based on the eref values, Sr atoms prefer the slightly larger Ba2-centred polyhedron with the < Ba2–O > distance of 2.70 Å; thus, the slightly smaller Ce3-centred polyhedron with the < Ce3–O > distance of 2.68 Å should be occupied predominantly by Ce atoms as it is too small for Ba atoms.
Table 5Selected interatomic distances (Å) in the structure of bainbridgeite-(YCe). The average interatomic distances are given in bold.
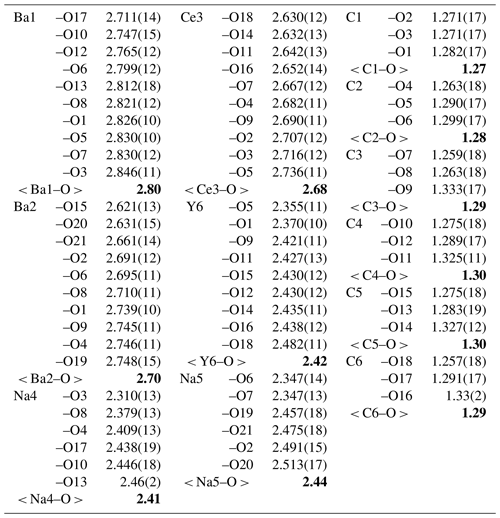
Table 6Refined site-scattering factors and assigned occupancies for Ba2, Ca3, and Y6 sites in the structure of bainbridgeite-(YCe)a.
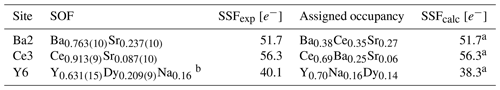
a SOF – refined site-occupation factor; SSFexp and SSFcalc – experimental and calculated site-scattering factors (numbers of electrons). b The occupancy of Na was fixed.
The preference of Ce atoms for the Ce3 site was also observed in alicewilsonite-(YCe) (Lykova et al., 2023b). The assigned occupancies are Ba0.38Ce0.35Sr0.27 and Ce0.69Ba0.25Sr0.06 for the Ba2 and Ce3 sites, respectively. Na4 and Na5 sites have octahedra coordination; their occupancies were fixed as Na0.93Dy0.07. The Y6 site is occupied predominantly by Y atoms (70 %) with admixed Na (16 %) and Dy (14 %) atoms. The Y6-centred nine-fold polyhedron has the < Y6–O > distance of 2.42 Å, which is similar to the < Y–O > distance observed in mckelveyite-(Y)-2M (2.41 Å; Demartin et al., 2008) and alicewilsonite-(YCe) (2.39 Å; Lykova et al., 2023b).
Table 7Comparative data for bainbridgeite-(YCe) and related mckelveyite group minerals.
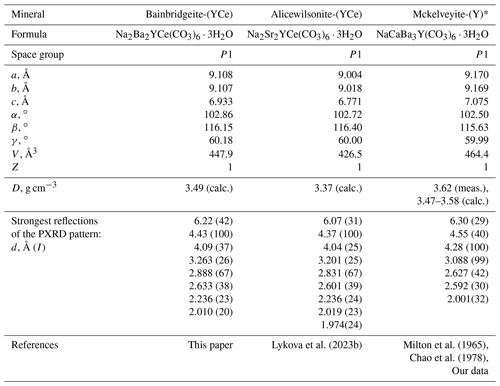
* Data for a triclinic polytype are given.
The arrangement of the six carbonate groups is similar to that of alicewilsonite-(YCe) (Lykova et al., 2023b) and weloganite (Grice and Perrault, 1975). The three CO groups centred by carbon atoms C1, C2, and C3 are almost coplanar with {001}, while the other three centred by C4, C5, and C6 atoms are not coplanar (Fig. 4).
The BVSs at the O19, O20, and O21 sites (0.34, 0.39, and 0.38 valence units, respectively) indicate the presence of H2O0 molecules, also confirmed by the presence of the bands of O–H stretching and H–O–H bending vibrations in the IR spectrum of bainbridgeite-(YCe) (Fig. 3). The three H2O molecules are bonded to Ba2- and Na5-centred polyhedra that share a face.
The resulting structural formula of bainbridgeite-(YCe) is (Na1.86Dy0.14)∑2.00(Ba1.38Ce0.35Sr0.27)∑2.00(Y0.70 Na0.16Dy0.14)∑1.00(Ce0.69 Ba0.25Sr0.06)∑1.00(CO3)6(H2O)3, which leads to the ideal formula Na2Ba2YCe(CO3)6 ⋅ 3H2O.
The structural formula is in a good agreement with the empirical formula (Na1.95HREE0.05)∑2.00(Ba1.33LREE0.30Sr0.27Ca0.10)∑2.00 (Y0.70Na0.16HREE0.14)∑1.00(LREE0.63Ba0.27Sr0.10)∑1.00 (CO3)5.86(H2O)3.00, where
LREE = Ce0.49La0.29Nd0.11Pr0.03Sm0.01, and
HREE = Dy0.07Er0.05Gd0.03Ho0.02Yb0.02.
Bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O, is a member of the mckelveyite group (Lykova et al., 2023a). It is the Ba analogue of alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, and the NaCe analogue of mckelveyite-(Y), NaCaBa3Y(CO3)6 ⋅ 3H2O (Table 7); the entry of Ce follows the coupled substitution scheme Ca Ba Na Ce3+.
Bainbridgeite-(YCe) and alicewilsonite-(YCe) form a continuous isomorphous series (Fig. 6), and phases with compositions intermediate between alicewilsonite-(YCe) and bainbridgeite-(YCe) are common. When the two minerals are found in one crystal, the composition can change both gradually and sharply, with a clear boundary between the phases. It is not unusual to find bainbridgeite-(YCe) and alicewilsonite-(YCe) in an epitactic relationships where either phase can be the host.
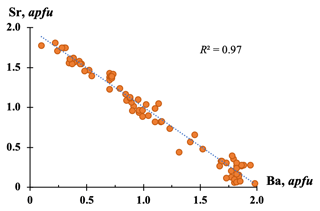
Figure 6Sr and Ba contents (apfu, calculated on the basis of six cations) in the members of alicewilsonite-(YCe) and bainbridgeite-(YCe) series (based on 82 EMPAs).
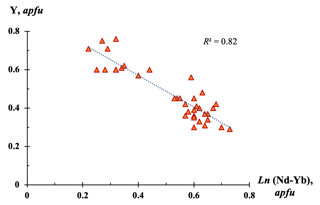
Figure 7Y and heavier Ln (Nd–Yb) contents (apfu, calculated on the basis of six cations) in bainbridgeite-(YCe) (based on 36 EMPAs).
On the other hand, bainbridgeite-(YCe) and mckelveyite-(Y) occur at Mont Saint-Hilaire as distinct mineral species, and we have not found phases with an intermediate composition. In all studied samples, the content of Ca in bainbridgeite-(YCe) does not exceed 0.23 apfu. Similar relationships were described between alicewilsonite-(YCe) and donnayite-(Y) from Mont Saint-Hilaire (Lykova et al., 2023b). Bainbridgeite-(YCe) and mckelveyite-(Y) are also rarely found in one crystal, although epitactic overgrowth of bainbridgeite-(YCe) on mckelveyite-(Y) has been observed.
Unlike in donnayite-(Y) and mckelveyite-(Y), where Sr and Ba atoms are distributed evenly among three large-cation sites (Demartin et al., 2008; Lykova et al., 2023a), in bainbridgeite-(YCe) Ba and Sr atoms show a preference for different sites. Therefore, a mineral with the general formula Na2SrBaYCe(CO3)6 ⋅ 3H2O with Ba, Sr, and Ce atoms ordered between three different cation sites could exist in nature.
Bainbridgeite-(YCe) is the second, after alicewilsonite-(YCe), example of crystal-chemically driven ordering of lighter and larger Ln at the larger cation sites and Y + heavier and smaller Ln at the smaller cation sites within one structure in the mckelveyite group minerals. Such ordering was previously described in davidite-(La), La(U,Y)Fe2(Ti,Fe,Cr,V)18(O,OH)38, reconstituted by heating (Gatehouse et al., 1979) and in tveitite-(Y), (Y,Na)6(Ca, LREE)6(Ca,Na,HREE)6(Ca,Na)F42 (Yakubovich et al., 2007).
A higher content of heavier Ln (up to 0.7 apfu), especially Nd, Sm, Gd, and Dy, and a lower Y content (as low as 0.2 apfu) were observed in some bainbridgeite-(YCe) samples (Table 1). The Ce and La contents in these phases remain relatively stable confirming the Y substitution scheme at the Y6 site and showing that Nd and Sm atoms can also selectively concentrate at that site, as it was also observed in alicewilsonite-(YCe) (Lykova et al., 2023a). A substantial correlation (R2= 0.82) observed between Y and heavier Ln contents in bainbridgeite-(YCe) based on 36 analyses (Fig. 7) confirms that the primary mechanism of entry of heavier and smaller Ln atoms into the structure is preferential concentration at the Y6 site.
Th is a common minor admixture in bainbridgeite-(YCe). Its entry could follow the Ca Y Na Th4+ substitution mechanism, but, as Th4+ cations are significantly larger than Y3+, the degree of tolerance of the bainbridgeite-(YCe) structure to Th cations is unclear.
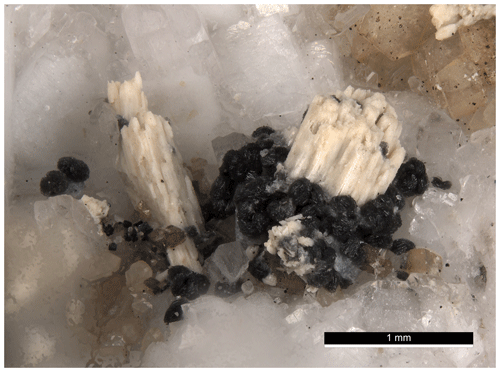
Figure 8Black rosettes of bainbridgeite-(YCe) forming a girdle around a pseudomorph of beige bastnaesite after remondite(?) from Mont Saint-Hilaire, Quebec, Canada. FOV 3.5 mm. CMNMC 91454. Canadian Museum of Nature collection. Photo: Michael Bainbridge.
The typical association of bainbridgeite-(YCe) and bastnaesite group minerals, which are often found in close proximity to one another and are sometimes intergrown, indicates that these minerals are often formed concurrently with late-stage alteration products of primary REE-bearing carbonates. Burbankite group minerals, for example remondite-(Ce), Na3(Ce,Ca,Na)3(CO3)5, or petersenite-(Ce), Na4(Ce,La,Nd)2(CO3)5, could be one such carbonate. This hypothesis is confirmed by the observation of pseudomorphs of bastnaesite after a burbankite group mineral with multiple rosettes of bainbridgeite-(YCe) forming girdles around the pseudomorphs (Fig. 8).
Crystallographic data for bainbridgeite-(YCe) and its PXRD pattern in the xy format are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-36-183-2024-supplement.
IL conceptualised the project. RR collected powder X-ray diffraction data and sub-sampled the specimens. EMPAs were obtained by GP. HF collected X-ray single-crystal diffraction data. KH obtained the IR spectrum. IL processed the data and interpreted the results. The manuscript was written by IL with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We would like to thank Jiří Sejkora and an anonymous referee for critical reading, Elsa Pfenninger-Horvath, Laszlo Horvath, and Peter Tarassoff for providing us with samples for the study, and François Génier and Michael Bainbridge for taking the colour photos.
This research was financially supported by the Canadian Museum of Nature.
This paper was edited by Sergey Krivovichev and reviewed by Jiří Sejkora and one anonymous referee.
Bayliss, P. and Levinson, A. A.: A system of nomenclature for rare-earth mineral species; revision and extension, Am. Mineral., 73, 422–423, 1988.
Chao, G. Y., Harris, D. C., Hounslow, A. W., Mandarino, J. A., and Perrault, G.: Minerals from the nepheline syenite, Mont Saint Hilaire, Quebec, Can. Mineral., 9, 109–123, 1967.
Chao, G. Y., Mainwaring, P. R., and Baker, J.: Donnayite, NaCaSr3Y(CO3)6 ⋅ 3H2O, a new mineral from Mont St-Hilaire, Quebec, Can. Mineral., 16, 335–340, 1978.
Chukanov, N. V. and Chervonnyi, A. D.: Infrared Spectroscopy of Minerals and Related Compounds, Springer Cham, Switzerland, 1109 pp., https://doi.org/10.1007/978-3-319-25349-7, 2016.
Donnay, G., Donnay, J. D. H., and Hey, M. H.: Ewaldite, a new barium calcium carbonate – I. Occurrence of ewaldite in syntactic intergrowth with mackelvyite, Tscher. Miner. Petrog., 15, 185–200, https://doi.org/10.1007/BF01087021, 1971.
Demartin, F., Gramaccioli, C. M., Campostrini, I., and Diella, V.: The crystal structure of mckelveyite-(Y)-2M, a new monoclinic polytype from Val Malenco, Italian Alps, Can. Mineral., 46, 195–203, https://doi.org/10.3749/canmin.46.1.195, 2008.
Gagné, O. C. and Hawthorne, F. C.: Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen, Acta Crystallogr. B, 71, 562–578, 10.1107/s2052520615016297, 2015.
Gatehouse, B. M., Grey, I. E., and Kelly, P. R.: The crystal structure of davidite, Am. Mineral., 64, 1010–1017, 1979.
Grice, J. D. and Perrault, G.: The crystal structure of triclinic weloganite, Can. Mineral., 13, 209–216, 1975.
Horvath, L., Gault, R. A., Pfenninger-Horvath, E., and Poirier, G.: Mont Saint-Hilaire: History, Geology, Mineralogy, The Canadian Mineralogist Special Publication 14, Mineralogical Association of Canada, Canada, 634 pp., 2019.
Levinson, A. A.: A system of nomenclature for rare-earth minerals, Am. Mineral., 51, 152–158, 1966.
Lykova, I., Rowe, R., Poirier, G., Giester, G., Ojaste, K., and Friis, H.: Mckelveyite group minerals – Part 1: Nomenclature and new data on donnayite-(Y), Eur. J. Mineral., 35, 133–142, https://doi.org/10.5194/ejm-35-133-2023, 2023a.
Lykova, I., Rowe, R., Poirier, G., Friis, H., and Helwig, K.: Mckelveyite group minerals – Part 2: Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, a new species, Eur. J. Mineral., 35, 143–155, https://doi.org/10.5194/ejm-35-143-2023, 2023b.
Milton, C., Ingram, B., Clark, J. R., and Dwornik, E. J.: Mckelveyite, a new hydrous sodium barium rare-earth uranium carbonate mineral from The Green River Formation, Wyoming, Am. Mineral., 50, 593–612, 1965.
Nespolo, M. and Ferraris, G.: Twinning by syngonic and metric merohedry. Analysis, classification and effects on the diffraction pattern, Z. Krist. – Cryst. Mater., 215, 77–81, https://doi.org/10.1524/zkri.2000.215.2.77, 2000.
Normand, C. and Tarassoff, P.: Mineralogy and geology of the Poudrette quarry, Mont Saint-Hilaire, Geological Association of Canada – Mineralogical Association of Canada, Guidebook, Field trip A4, 23 pp., 2006.
Rowe, R.: New statistical calibration approach for Bruker AXS D8 Discover microdiffractometer with Hi-Star detector using GADDS software, Powder Diffr., 24, 263–271, https://doi.org/10.1154/1.3193683, 2009.
Sabina, A. P., Jambor, J. L., and Plant, A. G.: Weloganite, a new strontium zirconium carbonate from Montreal Island, Canada, Can. Mineral., 9, 468–477, 1968.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr. C, 71, 3–8, https://doi.org/10.1107/s2053229614024218, 2015.
Yakubovich, O. V., Massa, W., Pekov, I. V., and Gavrilenko, P. G.: Crystal structure of tveitite-(Y): Fractionation of rare-earth elements between positions and the variety of defects, Crystallogr. Rep., 52, 71–79, https://doi.org/10.1134/S1063774507010087, 2007.
- Article
(9450 KB) - Full-text XML