the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mckelveyite group minerals – Part 4: Alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O, a new lanthanum-dominant species from the Paratoo mine, Australia
Inna Lykova
Ralph Rowe
Glenn Poirier
Henrik Friis
Kate Helwig
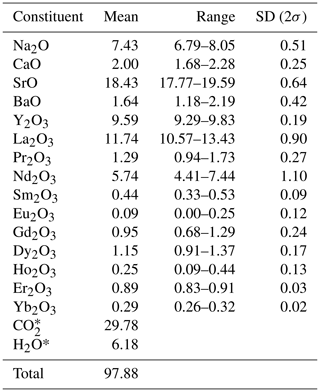
The new mckelveyite group mineral, alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O, was found together with kamphaugite-(Y), paratooite-(Y), bastnäsite-(La), and decrespignyite-(Y) coating along fractures in dolomite at the Paratoo copper mine, South Australia, Australia. It occurs as pale pink to colourless pseudohexagonal tabular crystals up to 150 µm in size. The streak is white; the lustre is vitreous. The mineral has no cleavage. Dcalc is 3.37 g cm−3. Alicewilsonite-(YLa) is optically biaxial (−), α = 1.556(2), β= 1.582(2), γ= 1.592(2), 2V (meas.) = 60(2)°, 2V (calc.) = 63° (589 nm). The IR spectrum is reported. The composition (wt %, average of seven analyses) is Na2O 7.43, CaO 2.00, SrO 18.43, BaO 1.64, Y2O3 9.59, La2O3 11.74, Pr2O3 1.29, Nd2O3 5.74, Sm2O3 0.44, Eu2O3 0.09, Gd2O3 0.95, Dy2O3 1.15, Ho2O3 0.25, Er2O3 0.89, Yb2O3 0.29, CO2 29.78, H2O 6.18, total 97.88. The empirical formula calculated on the basis of six cations with 3 H2O molecules is as follows: Na2.10Ca0.31Sr1.56Ba0.10Y0.74La0.63Pr0.07 Nd0.30Sm0.03Eu0.01Gd0.04Dy0.05Ho0.01Er0.04 Yb0.01(CO3)5.92(H2O)3. The mineral is triclinic, P1, a= 8.9839(2), b= 8.9728(3), c= 6.7441(2) Å, α= 102.812(2)°, β= 116.424(2)°, γ= 60.128(2)°, and V= 422.17(2) Å3 and Z= 1. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are 6.03(43)(001), 4.355(100)(10, 1, 120), 4.020(30)(11, 210, 1), 3.188(29)(2, 11, 121), 2.819(96)(002, 12, 211, 2), 2.592(40)(01, 030, 1), 2.228(33)(21, 1, 1). 2.011(36)(22, 003, 420, 2), 1.9671(32)(03, 301, 02, 032, 3, 331). The crystal structure was solved and refined from single-crystal X-ray diffraction data (R1= 0.058).
- Article
(3192 KB) - Full-text XML
- Companion paper 1
- Companion paper 2
- Companion paper 3
-
Supplement
(664 KB) - BibTeX
- EndNote
This paper describes alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O, the first lanthanum-dominant member of the mckelveyite group which consists of rare carbonates with the general formulaA3B3(CO3)6 ⋅ 3H2O, where A= Na, Ca, Y, and Zr, and B= Sr, Ba, Ce, and La (Lykova et al., 2023a).

Figure 1Transparent pale pink to colourless alicewilsonite-(YLa) crystals up to 150 µm in size, with white globular kamphaugite-(Y). FOV 1.5 mm. Specimen CMNMC 89063. Canadian Museum of Nature collection. Photos: François Génier.
Alicewilsonite-(YLa) is named as the La analogue of alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O. The parenthesised Levinson suffix -(YLa) was added in accordance with the nomenclature for rare-earth and Y mineral species (Levinson, 1966; Bayliss and Levinson, 1988) and the nomenclature of the mckelveyite group; the first symbol represents the dominant cations at one of the A sites and the second symbol at one of the B sites (Lykova et al., 2023a).
Both the new mineral and the name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA CNMNC), proposal IMA 2021-047. The holotype of alicewilsonite-(YLa) was deposited in the collection of the Canadian Museum of Nature, Ottawa, Canada. The catalogue number is CMNMC 89063. A part of the holotype used for structure determination is kept at the Natural History Museum in Oslo under catalogue number KNR 44330.
Alicewilsonite-(YLa) occurs at the Paratoo copper mine, Yunta, Olary Province, South Australia, Australia. It was found together with kamphaugite-(Y) as coating along fractures in dolomite. The new mineral forms pseudohexagonal tabular crystals up to 150 µm in size (Fig. 1). Similar crystals have been previously reported from Paratoo as “donnayite-(Y)” and are known to be associated with kamphaugite-(Y), paratooite-(Y), bastnäsite-(La) (Pring et al., 2006), and decrespignyite-(Y) (Wallwork et al., 2002). No analytical data were published on that phase, but electron microprobe analyses shared with us by Allan Pring (personal communication, 2023) correspond to alicewilsonite-(YLa). No specimen with conclusively identified donnayite-(Y) from the Paratoo mine is known to us.
At Paratoo, dolomites, sandstones, and shales of the Torrensian Burra Group were disrupted by several dolerite bodies and were brecciated forming the “Paratoo Diapir”. At its surface, the bulk of the copper mineralisation occurs in the quartz–magnetite veins, and as impregnations and fissure fillings of secondary copper minerals in sedimentary rocks. The secondary Cu-REE mineralisation, including alicewilsonite-(YLa), associated with the intensely weathered base metal and magnetite ores, occurs as thin coatings along bedding joints and fractures and is most abundant near the surface (< 3 m) (Brugger et al., 2006).
Alicewilsonite-(YLa) is pale pink to colourless with a white streak and vitreous lustre. The mineral has no cleavage, and its fracture is uneven. The Mohs hardness could not be determined as the crystals are very small; it is expected to be ca. 3, based on data for other members of the mckelveyite group. The mineral is non-fluorescent under ultraviolet light. The density calculated using the empirical formula and unit-cell volume refined from the single-crystal XRD data is 3.37 g cm−3.
Alicewilsonite-(YLa) is optically biaxial (−), α= 1.556(2), β= 1.582(2), γ= 1.592(2), 2 V (meas.) = 60(2)° (from a spindle-stage extinction curve), 2 V (calc.) = 63°. The mineral is non-pleochroic.
Electron microprobe analyses (EMPAs) for alicewilsonite-(YLa) were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five WDS spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) using an accelerating voltage of 20 kV, a beam current of 10 nA, and a beam diameter of 30 µm depending on the grain size. Alicewilsonite-(YLa) is unstable under an electron beam, and so a large beam diameter was used to minimise element migration. The following reference materials were used: albite (NaKα), diopside (CaKα), celestine (SrLα), sanbornite (BaLα), YAG (YLα), LaPO4 (LaLα), PrPO4 (PrLβ), NdPO4 (NdLα), SmPO4 (SmLα), EuPO4 (EuLα), GdPO4 (GdLα), DyPO4 (DyLβ), HoPO4 (HoLβ), ErPO4 (ErLα), (YbLα). The intensity data were corrected for time-dependent intensity loss (or gain) using a self-calibrated correction for NaKα, CaKα, YLα, and LaLα. H2O and CO2 contents were not analysed due to the paucity of the available material.
The Fourier transform infrared (FTIR) spectrum of alicewilsonite-(YLa) was obtained using a Bruker Hyperion 2000 microscope interfaced to a Tensor 27 spectrometer with a wide-band mercury cadmium telluride (MCT) detector (Canadian Conservation Institute, Canada). A small fragment of alicewilsonite-(YLa) was mounted on a low-pressure diamond anvil microsample cell and analysed in transmission mode. The spectrum was collected between 4000–400 cm−1 with the co-addition of 150 scans at a 4 cm−1 resolution.
Powder X-ray diffraction (PXRD) data were collected at the Canadian Museum of Nature, Canada, using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and IμS microfocus X-ray source (λCuKα1= 1.54060 Å) with the Kα2 contribution removed using the “Strip Kα2” tool in Bruker Diffrac.EVA V4.3. The instrument was calibrated using a statistical calibration method (Rowe, 2009). A powder ball of alicewilsonite-(YLa) ∼ 200 µm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10° rocking motion along the Psi axis of the Centric Eulerian Cradle stage.
Single-crystal X-ray diffraction (SXRD) studies were carried out at the Natural History Museum, University of Oslo, Norway, using a Rigaku XtaLAB Synergy-S diffractometer equipped with a HyPix 6000HE detector (λMoKα= 0.71073) operating at 50 kV and 1 mA. The data were processed, including absorption correction, using Rigaku's CrysAlis Pro software (Matsumoto et al., 2021).
5.1 Chemical data
Chemical data on alicewilsonite-(YLa) are given in Table 1. The contents of Ce, Tb, Tm, U, Th, and Hf are below the detection limit.
The empirical formula calculated on the basis of six cations with 3 H2O molecules is Na2.10Ca0.31Sr1.56Ba0.10Y0.74La0.63Pr0.07Nd0.30 Sm0.03Eu0.01Gd0.04Dy0.05Ho0.01Er0.04Yb0.01 (CO3)5.92(H2O)3. The simplified formula is Na2(Sr,Ca,Ba)2(Y,Dy,Er)(La,Nd,Pr)(CO3)6 ⋅ 3H2O.
The ideal end-member formula is Na2Sr2YLa(CO3)6 ⋅ 3H2O, which requires Na2O 7.18, SrO 24.01, Y2O3 13.08, La2O3 18.88, CO3 30.59, H2O 6.26, total 100 wt %.
Alicewilsonite-(YLa) dissolves in an aqueous HCl solution at room temperature with strong effervescence.
5.2 Infrared spectroscopy
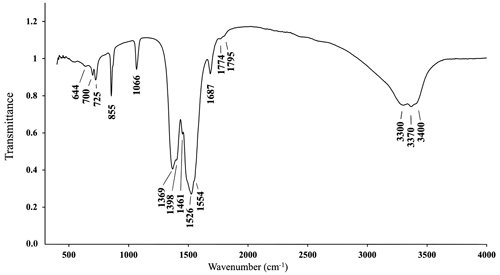
The IR spectrum of alicewilsonite-(YLa) (Fig. 2) shows IR bands of O–H stretching (in the range from 3300–3400 cm−1) and H–O–H bending (at 1687 cm−1) vibrations of H2O molecules and C–O stretching (in the range 1369–1554 cm−1) vibrations of CO group molecules. The band at 1066 cm−1 can be assigned to the non-degenerate mode of C–O stretching vibrations, indicating polarisation of CO groups. If only symmetric non-polarised carbonate groups (with a 3-fold axis) were present, this mode would be inactive. The band at 855 cm−1 could correspond to the non-degenerate mode of out-of-plane O–C–O bending vibrations, and its high intensity may be attributed to a high degree of distortion of the CO groups. The band assignment was made after Chukanov and Chervonnyi (2016) and Kasatkin et al. (2021).
5.3 X-ray diffraction data and description of the crystal structure
The indexed PXRD data are given in Table 2. Parameters of the triclinic unit cell refined from the data are as follows: a= 8.9839(2), b= 8.9728(3), c= 6.7441(2) Å, α= 102.812(2)°, β= 116.424(2)°, γ= 60.128(2)°, and V= 422.17(2) Å3. The PXRD pattern in the xy format is available as a Supplement.
Table 2X-ray powder diffraction data (d in Å) for alicewilsonite-(YLa). The strongest reflections are given in bold.
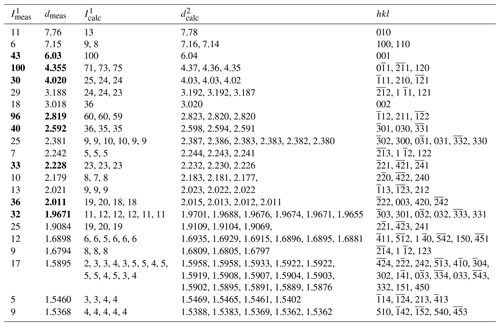
1 Calculated from the crystal structure determination; only reflections with intensities > 1 are given. 2 Calculated from PXRD Rietveld unit-cell refinement with a= 8.9839(2), b= 8.9728(3), c= 6.7441(2) Å, α = 102.812(2)°, β= 116.424(2)°, γ = 60.128(2)°, and V= 422.17(2) Å3.
The SXRD data were indexed in the P1 space group with the following unit-cell parameters: a= 8.9793(3) Å, b= 8.9734(2) Å, c= 6.74044(19) Å, α= 102.829(2)°, β= 116.382(3)°, γ= 60.109(3)°, and V= 421.82(2) Å3. The structure was solved and refined to R1= 0.058 on the basis of 3640 independent reflections with I>2σ(I) using the SHELXL-2018/3 program package (Sheldrick, 2015). Crystal data, data collection information, and structure refinement details are given in Table 3, atom coordinates, equivalent displacement parameters, site composition, and bond-valence sums (BVSs) in Table 4, and selected interatomic distances in Table 5. The studied crystal demonstrated twinning by merohedry Class I (Nespolo and Ferraris, 2000), with twin domains ratio of 22:78. The crystallographic information file (CIF) for alicewilsonite-(YLa) is available as a file in the Supplement. It was also deposited in the Inorganic Crystal Structure Database (ICSD; no. 2308960).
Table 3Crystal data, data collection information, and structure refinement details for alicewilsonite-(YLa).
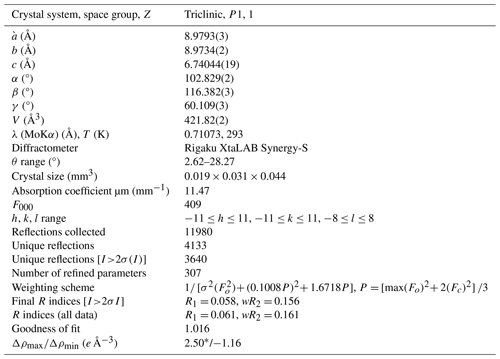
* Located 0.14 Å away from the Sr1 site.
Table 4Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms, site occupancies, and bond-valence sums (BVSs) for alicewilsonite-(YLa).
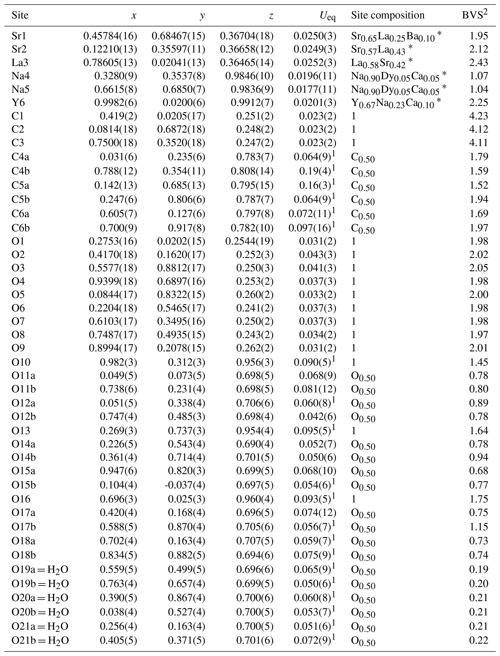
1 Uiso. 2 Bond-valence parameters were taken from Gagne and Hawthorne (2015). * The sites occupancies were refined assuming full occupancy; the best agreement was obtained with Sr0.617(7)La0.383(7) for the Sr1 site, Sr0.541(7)La0.459(7) for the Sr2 site, La0.584(8)Sr0.416(8) for the La3 site, Na0.918(7)Dy0.082(7) for the Na4 site, Na0.916(6)Dy0.084(6) for the Na5 site, and Y0.716(16)Na0.284(16) for the Y6 site. In the final refinement cycles the occupancies were fixed based on the eref values, electron microprobe data, interatomic distances, bond-valence calculations, and the charge balance.
Table 5Selected interatomic distances (Å) in the structure of alicewilsonite-(YLa).
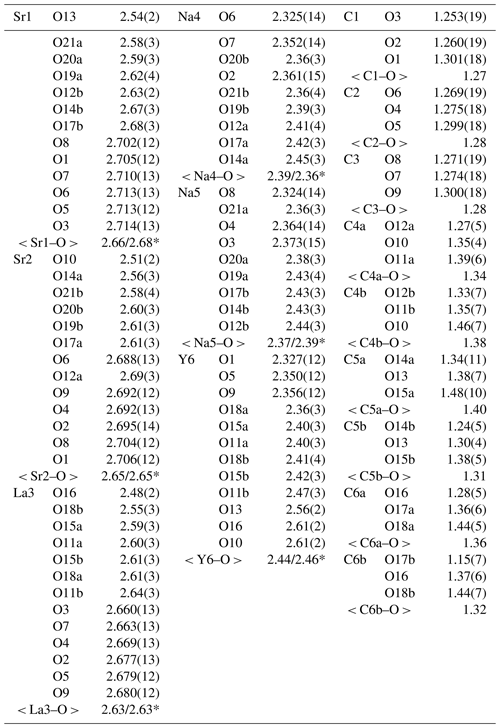
* Calculated for polyhedra with bonds M–O sites marked with appended “a” / M–O sites marked with appended “b”.
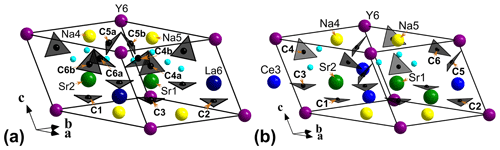
Due to the poor quality of alicewilsonite-(YLa) crystals resulting in multiple split reflections and streaks, the structure could only be refined to R1= 0.058. The mineral is strongly pseudotrigonal. There are six independent large cation sites in the structure (Fig. 3a) forming two alternating layers parallel to the ab plane (Fig. 4). Na, Sr, Y, La, Ca, Dy, and Ba cations were distributed among these sites based on the microprobe data, refined site-scattering factors (eref, in electrons per site), and charge balance taking into account bond-valence sums (BVSs) and interatomic distances (Tables 4–5). Lighter lanthanoids (Ln, La–Lu) were formally refined as La atoms and heavier Ln as Dy atoms. One of the layers is formed by the Sr1, Sr2, and La3 sites that have a 10-fold coordination. The refinement showed that Sr and La are distributed between all three sites; the best agreement was obtained with the refined site-occupation factor Sr0.617(7)La0.383(7) for the Sr1 site, Sr0.541(7)La0.459(7) for the Sr2 site, and La0.584(8)Sr0.416(8) for the La3 site. The occupancies were assigned as Sr0.65La0.25Ba0.10, Sr0.57La0.43, and La0.58Sr0.42 for the Sr1, Sr2, and La3 sites, respectively. Na4 and Na5 sites have octahedra coordination; their occupancies were fixed as Na0.90Dy0.05Ca0.05. The Y6 site is occupied predominantly by Y atoms (67 %) with admixed Na (23 %) and Ca (10 %) atoms. The Y6-centred nine-fold polyhedron has an average < Y6–O > distance of 2.44/2.46 Å.
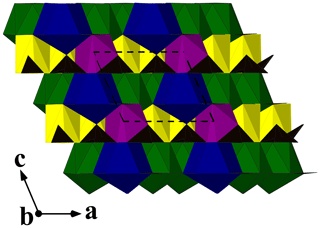
Figure 4A view along [010] of the crystal structure of alicewilsonite-(YLa). Sr-, La-, Y-, and Na-centred polyhedra are shown in green, blue, purple, and yellow, respectively. Carbonate groups are black triangles. The unit cell is outlined.
The three CO groups centred by carbon atoms C1, C2, and C3 are almost coplanar with {001} as in alicewilsonite-(YCe) (Lykova et al., 2023b). In the latter, as well as in most other member of the mckelveyite group (Lykova et al., 2023a, 2024), there are three other, not coplanar CO groups centred by carbon atoms C4, C5, and C6 (Fig. 3b). In alicewilsonite-(YLa) these three sites are split into two alternating sites each marked by letters a and b, e.g. C4a and C4b, forming alternating CO groups with a shared vertex (Fig. 3a). The occupancy of the alternating carbon and oxygen sites was fixed at 50 %. Similar splitting of carbon sites was observed in donnayite-(Y) (Lykova et al., 2023a).
The BVSs at the O19a, O19b, O20a, O20b, O21a, and O21b sites (0.19, 0.20, 0.21, 0.21, 0.21, and 0.22 valence units, respectively) indicate the presence of H2O0 molecules, also confirmed by the presence of the bands of O–H-stretching and H–O–H bending vibrations in the IR spectrum of alicewilsonite-(YLa). O19a, O20a, and O21a water molecules are bonded to Sr1- and Na5-centred polyhedra that share a face, while O19b, O20b, and O21b water molecules are bonded to Sr2- and Na4-centred polyhedra.
Only one group of alternating C and O atoms marked by either “a” or “b” is occupied at the same time. Due to the quality of the collected data these atoms were not very reliably localised, and most were refined with isotropic atomic displacement parameters only.
The resulting structural formula of alicewilsonite-(YLa) is (Na1.80Dy0.10 Ca0.10)∑2 (Sr1.22La0.68Ba0.10)∑2(Y0.67Na0.23Ca0.10)∑1 (La0.58Sr0.42)∑1(CO3)6(H2O)3, which leads to the ideal formula Na2Sr2YLa(CO3)6 ⋅ 3H2O. Combining structural and chemical data results in the following formula: (Na1.90HREE0.10)∑2(Sr1.14LREE0.51Ca0.25Ba0.10)∑2 (Y0.74Na0.20Ca0.06)∑1(LREE0.52Sr0.42HREE0.06)∑1 (CO3)5.92(H2O)3, where LREE = La0.63Nd0.30Pr0.07Sm0.03, and HREE = Dy0.05Gd0.04Er0.04Eu0.01Ho0.01Yb0.01.
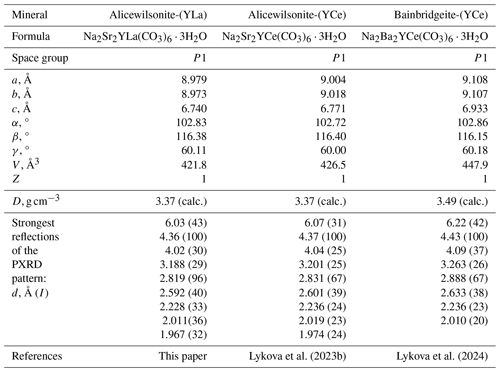
Alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O, is a member of the mckelveyite group (Lykova et al., 2023a). It is the La analogue of alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O (Lykova et al., 2023b), and the SrLa analogue of bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O (Lykova et al., 2024; Table 6).
The absence of Ce in alicewilsonite-(YLa) suggests that it was separated and deposited elsewhere as highly insoluble Ce4+. Whole-rock geochemical analyses of fresh and mineralised host rock and of vein material at Paratoo conducted by Brugger et al. (2006) revealed a strong enrichment in light rare-earth elements (LREE) and a strong negative Ce anomaly in secondary REE minerals and mineralised rock samples. This indicates that Ce was present as Ce4+ and left behind during either the transport from the unknown source rock to the Paratoo deposit or the deep weathering and remobilisation of REE elements. That the latter may result in preferential leaching of trivalent REE was demonstrated by Taunton et al. (2000) in a granite. The fractionation of Ce4+ led to the formation of La-dominant secondary REE carbonates, such as paratooite-(La), (La,Sr,Ca)4CuCa(Na,Ca)2(CO3)8 (Pring et al., 2006; Krivovichev et al., 2019), bastnäsite-(La), La(CO3)F (Pring et al., 2006), and alicewilsonite-(YLa).
We have previously shown the penchant of the mckelveyite structure type to incorporate lighter and larger Ln at the larger cation sites and Y+ heavier and smaller Ln at the smaller cation sites (Lykova et al., 2023a, b, 2024). Alicewilsonite-(YLa) is another example of such crystal-chemically driven ordering and the first one with La as the dominant cation at one of the sites.
The splitting of carbon sites observed in donnayite-(Y) (Lykova et al., 2023a) and alicewilsonite-(YLa) likely represents structural variations within one species and is expected to be found in other mckelveyite group minerals. In fact, we anticipate it to be relatively common, but the poor quality of crystals in this group resulting in multiple split reflections and streaks in SXRD data sets often does not allow for a proper localisation of half-occupied carbon and oxygen sites. Thus, the structures of these minerals are usually solved on crystals where the splitting is not observed.
Crystallographic data for alicewilsonite-(YLa) and its PXRD pattern in the xy format are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-36-301-2024-supplement.
IL conceptualised the project. RR collected powder X-ray diffraction data. EMPAs were obtained by GP. HF collected single-crystal X-ray diffraction data. KH obtained the IR spectrum. IL processed the data and interpreted the results. The manuscript was written by IL with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We would like to thank Ulf Hålenius and Allan Pring for critical reading, Elsa Pfenninger-Horvath and Laszlo Horvath for providing us with the sample for the study, Allan Pring for sharing additional information on mineralogy of the Paratoo mine, and François Génier for taking the colour photo.
This research was financially supported by the Canadian Museum of Nature.
This paper was edited by Sergey Krivovichev and reviewed by Ulf Hålenius and Alan Pring.
Bayliss, P. and Levinson, A. A.: A system of nomenclature for rare-earth mineral species; revision and extension, Am. Mineral., 73, 422–423, 1988.
Brugger, J., Ogierman, J., Pring, A., Waldron, H., and Kolitsch, U.: Origin of the secondary REE-minerals at the Paratoo copper deposit near Yunta, South Australia, Mineral. Mag., 70, 609–627, 2006.
Chukanov, N. V. and Chervonnyi, A. D.: Infrared Spectroscopy of Minerals and Related Compounds, Springer Cham, Switzerland, 1109 pp., https://doi.org/10.1007/978-3-319-25349-7, 2016.
Gagne, O. C. and Hawthorne, F. C.: Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen, Acta Crystallogr. B, 71, 562–578, https://doi.org/10.1107/s2052520615016297, 2015.
Kasatkin, A. V., Zubkova, N. V., Pekov, I. V., Chukanov, N. V., Škoda, R., Agakhanov, A. A., Belakovskiy, D. I., Britvin, S. N., and Yu. Pushcharovsky, D.: The mineralogy of the historical Mochalin Log REE deposit, South Urals, Russia. Part IV. Alexkuznetsovite-(La), La2Mn(CO3)(Si2O7), alexkuznetsovite-(Ce), Ce2Mn(CO3)(Si2O7) and biraite-(La), La2Fe2+(CO3)(Si2O7), three new isostructural minerals and a definition of the biraite group, Mineral. Mag., 85, 772–783, https://doi.org/10.1180/mgm.2021.64, 2021.
Krivovichev, S.V., Panikorovskii, T.L., Zolotarev, A.A., Bocharov, V.N., Kasatkin, A.V., and Škoda, R.: Jahn-teller distortion and cation ordering: the crystal structure of paratooite-(La), a superstructure of carbocernaite, Minerals, 9, 1–11, https://doi.org/10.3390/min9060370, 2019.
Levinson, A. A.: A system of nomenclature for rare-earth minerals, Am. Mineral., 51, 152–158, 1966.
Lykova, I., Rowe, R., Poirier, G., Giester, G., Ojaste, K., and Friis, H.: Mckelveyite group minerals – Part 1: Nomenclature and new data on donnayite-(Y), Eur. J. Mineral., 35, 133–142, https://doi.org/10.5194/ejm-35-133-2023, 2023a.
Lykova, I., Rowe, R., Poirier, G., Friis, H., and Helwig, K.: Mckelveyite group minerals – Part 2: Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, a new species, Eur. J. Mineral., 35, 143–155, https://doi.org/10.5194/ejm-35-143-2023, 2023b.
Lykova, I., Rowe, R., Poirier, G., Friis, H., and Helwig, K.: Mckelveyite group minerals – Part 3: Bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O, a new species from Mont Saint-Hilaire, Canada, Eur. J. Mineral., 36, 183–194, https://doi.org/10.5194/ejm-36-183-2024, 2024.
Matsumoto, T., Yamano, A., Sato, T., Ferrara, J. D., White, F. J., and Meyer, M.: “What is This?” A structure analysis tool for rapid and automated solution of small molecule structures, J. Chem. Crystallogr., 51, 438–450, https://doi.org/10.1007/s10870-020-00867-w, 2021.
Nespolo, M. and Ferraris, G.: Twinning by syngonic and metric merohedry. Analysis, classification and effects on the diffraction pattern, Z. Krist.-Cryst. Mater., 215, 77–81, https://doi.org/10.1524/zkri.2000.215.2.77, 2000.
Pring, A., Wallwork, K., Brugger, J., and Kolitsch, U.: Paratooite-(La), a new lanthanum-dominant rare-earth copper carbonate from Paratoo, South Australia, Mineral. Mag., 70, 131–138, 2006.
Rowe, R.: New statistical calibration approach for Bruker AXS D8 Discover microdiffractometer with Hi-Star detector using GADDS software, Powder Diffr., 24, 263–271, https://doi.org/10.1154/1.3193683, 2009.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr. C, 71, 3–8, https://doi.org/10.1107/s2053229614024218, 2015.
Taunton, A. E., Welch, S. A., and Banfield, J. F.: Microbial controls on phosphate and lanthanide distributions during granite weathering and soil formation, Chem. Geol., 169, 371–382, https://doi.org/10.1016/S0009-2541(00)00215-1, 2000.
Wallwork, K., Kolitsch, U., Pring, A., and Nasdala, L.: Decrespignyite-(Y), a new copper yttrium rare earth carbonate chloride hydrate from Paratoo, South Australia, Mineral. Mag., 66, 181–188, https://doi.org/10.1180/0026461026610021, 2002.
- Article
(3192 KB) - Full-text XML