the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
High-pressure behaviour of a CaIrO3 perovskite-type structure
Tiziana Boffa Ballaran
Kanchana Kularatne
Reidar G. Trønnes
Structural refinements of single-crystal X-ray diffraction data collected at room temperature both under ambient and under high pressures up to 10 GPa have been used to characterise the octahedral tilting and site distortions of the orthorhombic Pbnm CaIrO3 perovskite-type phase. The octahedral tilting has been evaluated using both the unit-cell lattice parameters and the displacements of the oxygen and A site from their hypothetical positions in an ideal cubic perovskite structure. The difference between the two types of calculations shows that the assumption of rigid and undistorted octahedra made for determining the tilt angles from the unit-cell parameters is valid only as a first approximation, especially in the case of CaRuO3, CaRhO3 and CaIrO3 perovskites, for which polyhedral distortions are significant. CaIrO3 perovskite appears to have much larger tilting and distortion than other CaO perovskites, likely causing the structure to be stiffer than expected from the general trend of the bulk moduli defined by other CaO perovskites. Moreover, in contrast to other CaO perovskites for which the Ca polyhedron is more compressible than the octahedral site, the compressibility of the IrO6 octahedra is very similar to that of the CaO12 polyhedra, giving rise to an octahedral tilting that is independent of pressure at least up to 10 GPa. This behaviour may help reduce the electronic interactions among oxygens of neighbouring octahedra during compression, given the already large tilts present in the CaIrO3 perovskite structure, and it is likely responsible for the bulk modulus's first pressure derivative being smaller than 4. Larger tilting and distortions are also observed for CaO perovskites that have a Pt-group element occupying the octahedral site. Such perovskites have been found to undergo a phase transformation to an orthorhombic Cmcm post-perovskite phase (CaIrO3-type structure) at pressures below 25 GPa, suggesting that a large octahedral tilting and polyhedral distortion may be a requisite for stabilising the orthorhombic post-perovskite phase of CaO perovskite-type materials.
- Article
(1194 KB) - Full-text XML
-
Supplement
(304 KB) - BibTeX
- EndNote
The discovery of a phase transition from orthorhombic Pbnm MgSiO3 bridgmanite to an orthorhombic post-perovskite Cmcm phase (Murakami et al., 2004; Oganov and Ono, 2004) sparked major interest in the behaviour of CaIrO3, which is isostructural with post-perovskite under ambient conditions and transforms to a perovskite-type structure at 1–3 GPa and temperatures > 1350 °C (McDaniel and Schneidler, 1972; Hirose and Fujita, 2005; Kojitani et al., 2007a; Stølen and Trønnes, 2007; Trønnes et al., 2006) and therefore can be used as an analogue of the high-pressure MgSiO3 phases. However, the majority of studies have focused their attention on the behaviour of the Cmcm CaIrO3 phase, whereas only one study (Cheng et al., 2011) has reported the structural data of the CaIrO3 perovskite-type phase (CaIrO3 pv) obtained from synchrotron X-ray powder diffraction. CaIrO3 pv exhibits an orthorhombic Pbnm symmetry like many other CaO perovskites, which displays different degrees of distortion from the cubic aristotype perovskite structure Pm. This distortion is described in terms of octahedral tilting about the pseudo-cubic axes (Glazer, 1972). Several methods have been used in the literature to calculate the tilt angles of the orthorhombic perovskite structure. The simplest way is to make use of unit-cell lattice parameters (i.e. Mitchell, 2002; O'Keeffe et al., 1979; Zhao et al., 1993) under the assumption that the octahedra are rigid and undistorted and that the orthorhombic distortion is therefore due to only octahedral tilting. However, since the octahedral framework in orthorhombic perovskite is neither rigid nor regular, a more accurate description of the octahedral tilting and polyhedral distortion of the orthorhombic perovskite structure is obtained from symmetry-mode analysis with respect to the ideal cubic perovskite structure. Symmetry modes are collective correlated atomic displacements that follow specific symmetry properties (Perez-Mato et al., 2010). The structural distortion in orthorhombic perovskites can be decomposed into contributions from different modes with symmetries given by the irreducible representations (Irreps) of the cubic Pm aristotype structure (Howard and Stokes, 2002, 1998; Perez-Mato et al., 2010). Such decomposition allows the octahedral tilting to be separated from the B- and A-site distortions. Only Irreps at the following k points of the reciprocal space of the cubic perovskite needs to be considered: Γ (0, 0, 0), X (0, 0, ), M (, , 0) and R (, , ). Using the notation of Miller and Love (1967), Howard and Stokes (1998, 2002) have shown that the two Irreps and induce the out-of-phase and in-phase tilt patterns of the perovskite octahedral framework and that they are truly independent of the polyhedral distortion. For Pbnm perovskites, three more Irreps are indicated as secondary symmetry modes, since they do not drive the displacive phase transformation from the cubic to the orthorhombic structure. These modes (, and ) usually have much smaller amplitudes with respect to the and primary modes and are responsible for the polyhedral distortion in orthorhombic perovskite. Moreover, whilst the , and modes are related only to displacements of the oxygen atoms, the and modes are due to displacements of both the oxygen atoms and the Ca atoms occupying the A site. A sketch visualisation of the displacements of the oxygens and A-site cations from their ideal position in the cubic aristotype structure associated with the five symmetry-adapted modes is given in Huang et al. (2021).
For CaB4+O3 perovskites, with B representing the cation occupying the octahedral B site, we can expect that octahedral tilting and distortion vary systematically with the size of the B-site cations, since Ca always occupy the cavity (A site) of the three-dimensional octahedral framework. This hypothesis is supported by the fact that the variation in the isothermal bulk modulus (KT0) of CaB4+O3 perovskites with the B cation being Zr, Sn, Ti and Ge falls on a single smooth trend versus the inverse of the molar volume (Ross and Chaplin, 2003), implying that for larger unit-cell volumes (i.e. at lower atom packing densities), the bulk modulus is lower and the perovskites are softer. However, the bulk modulus of CaIrO3 pv determined both from compression experiments (Boffa Ballaran et al., 2007) and from ab initio calculations (Marbouh et al., 2021) is much higher than that of other CaO perovskites and plots above the trend reported by Ross and Chaplin (2003). Moreover, the first pressure derivative of the bulk modulus, K′, is smaller than 4, whereas the other CaO perovskites have K′ values close to 6. In order to shed light on the structural reasons for the anomalous stiffening of CaIrO3 perovskite with respect to the other CaB4+O3 perovskite, we performed a high-pressure diffraction study on a single crystal of CaIrO3 pv and obtained the variation in the crystal structure of this perovskite up to 10 GPa.
2.1 Single-crystal X-ray diffraction under ambient conditions and structural refinements
A single crystal of CaIrO3 pv with dimensions µm3 (Ba8x2) was selected from the run product synthesised in a previous study (Trønnes et al., 2006) at 1525 °C and 2.5 GPa in a piston-cylinder apparatus from a starting mixture of high-purity CaCO3 and IrO2 decarbonated and sintered at 900–950 °C. A crystal from the same run product was used in the compressibility study performed by Boffa Ballaran et al. (2007). Full-intensity data collection for structural refinements under ambient conditions was performed using the Oxford Diffraction Xcalibur diffractometer equipped with a graphite monochromator; a ceramic X-ray tube with Mo radiation, operated at 50 kV and 40 mA; and a Sapphire 2 CCD detector. Complete and redundant intensity data were collected using ω scans with a width of 0.5° and a default time of 15 s up to 2θ=72.5°. The intensity data were integrated using CrysAlisPro software (Oxford Diffraction, Rigaku), applying Lorentz and polarisation corrections. Given the large linear absorption coefficient of CaIrO3 pv (μ=60.05 mm−1 for Mo radiation), an analytical absorption correction based on the crystal shape (Clark and Reid, 1995) was also performed together with an empirical correction, taking into account frame and detector area scaling. A total of 568 unique reflections were obtained, of which 526 have I > 2σ(I), with a final discrepancy factor Rint=0.0557. Systematic absences are consistent with the Pbnm space group, which has therefore been used to solve the CaIrO3 pv structure using the SHELXS programme (Sheldrick, 2015). The Ir and Ca atomic positions were identified using the Patterson algorithm, whereas the oxygen positions in the asymmetric unit were found by analysing the difference electron density map. Structural refinements were performed using the SHELXL programme (Sheldrick, 2015) built in the ShelXle (Hübschle et al., 2011) graphical user interfaces. Neutral scattering factors for Ca, Ir and O were used, and all atoms were refined anisotropically. The crystallographic information file (CIF) is available in the Supplement.
2.2 High-pressure single-crystal X-ray diffraction and structural refinements
Unfortunately, the Ba8x2 crystal was lost while trying to cut it to produce a smaller crystal suited for the high-pressure experiment. Therefore, another crystal (Ba8x4) with dimensions µm3 was selected. A BX90-type diamond anvil cell (DAC) (Kantor et al., 2012), with diamond culets of 400 µm diameter glued into Boehler–Almax seats with an opening angle of 90°, was prepared with a Re gasket pre-indented to a thickness of ∼ 60 µm from its original thickness of 200 µm. A hole with a diameter of 200 µm was laser-cut into the centre of this indentation to produce the sample chamber. The CaIrO3 crystal was loaded inside the DAC together with a small ruby sphere as a pressure calibrant (Shen et al., 2020). Neon gas was loaded into the DAC to act as a pressure-transmitting medium, using the gas-loading system installed at the BGI (Kurnosov et al., 2008). The DAC was left to stabilise after each pressure increase for at least 1 d before performing the XRD measurements. The ruby luminescence was measured before and after each XRD measurement by means of a Horiba LABRAM HR Raman micro-spectrometer equipped with an 1800 gr mm−1 grating and a He–Ne laser (λ=632.8 nm) with 20 mW laser power.
Intensity data for all accessible reflections were collected at room temperature and at six different pressure points, including ambient pressure in the DAC without a pressure medium up to 9.7 (1) GPa. Above this pressure, significant broadening of the reflections was observed, and therefore the experiment was interrupted. Data collection at different pressures was performed using ω scans with a step size of 1° in a fixed-φ mode (Finger and King, 1978) up to 63° in 2θ. Every frame was measured for 120 s. The intensity integration was performed using CrysAlisPro software (Oxford Diffraction, Rigaku), applying Lorentz and polarisation corrections. Absorption effects due to the sample and diamonds, as well as shadowing effects due to the gasket, have been taken into account using the Absorb programme (Angel, 2004) implemented in the CrysAlisPro software. Due to the DAC spatial limitation, the resulting number of unique reflections was 241 for the crystal in the DAC under ambient pressure and between 210 and 230 for the collections performed under high pressures. Structural refinements were performed using the SHELX programmes (Sheldrick, 2015) built in the ShelXle (Hübschle et al., 2011) graphical user interface, using the starting parameters obtained in this study under ambient conditions. Neutral scattering factors for Ca, Ir and O were used, and, due to the limited number of unique reflections, all atoms were refined isotropically. Difference Fourier maps show some residual electron density around the Ir atom, given the high electronic number of this atom and the use of an isotropic model. For this reason, uncertainties regarding the oxygen positions are larger than those obtained under ambient conditions but are still sufficiently precise for constraining the high-pressure compression of the CaIrO3 pv structure. A CIF containing all the high-pressure refinements of crystal Ba8x4 is available in the Supplement.
3.1 Structural features of CaB4+O3 perovskites under ambient conditions
The constrained unit-cell lattice parameters of the Ba8x2 sample obtained from a total of 2158 non-unique reflections (Table 1) are within 2σ from those published for another crystal of CaIrO3 pv taken from the same run product (Boffa Ballaran et al., 2007), obtained using the accurate eight-position centring method (Angel et al., 2000; King and Finger, 1979) with a point detector. The structural data obtained in this study are also in good agreement with those obtained from Rietveld refinements of synchrotron powder diffraction data (Cheng et al., 2011).
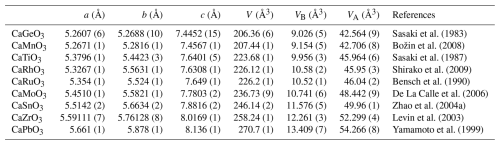
The octahedral volumes, VB, of CaIrO3 pv and of several CaB4+O3 perovskites follow, as expected, a linear trend as a function of the ionic radius (Shannon and Prewitt, 1969) of the cation occupying the octahedral site (Fig. 1), increasing from CaGeO3 up to the large CaPbO3 (Table 2). CaIrO3 and, to a lesser extent, CaRhO3 deviate slightly from the trend, though. This deviation is also visible in the variation in the VB values with the molar volume, Vmol (Fig. 2), whereas the volume of the A site, VA, defined as the volume of the unit cell normalised to the number of formula units minus the volume of the B site (), of all the CaB4+O3 perovskites, including CaIrO3, varies linearly as a function of Vmol (Fig. 2).
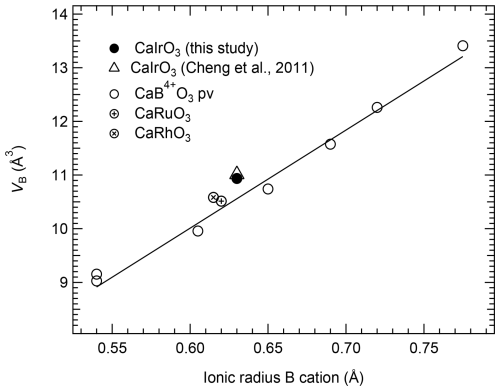
Figure 1Linear correlation between the octahedral volume, VB, and the ionic radius of the cation occupying the B site for several CaB4+O3 pv (Table 2) and for CaIrO3 pv investigated in this study and in the study from Cheng et al. (2011). The solid line is a linear weighed fit through the CaB4+O3 perovskite data reported in Table 2. The uncertainties are smaller than the symbol size.
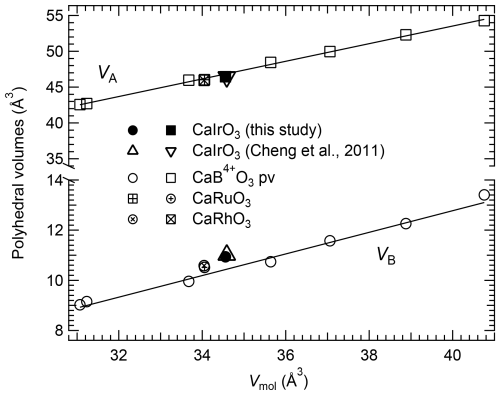
Figure 2Linear correlation of the octahedral volume, VB (circles), and the volume of the A site, VA (squares), with the molar volume, Vmol, of several CaB4+O3 pv. The VB values of CaIrO3 deviate slightly from the linear trend. Solid lines are linear weighed fits through the CaB4+O3 perovskite data reported in Table 2. The uncertainties are smaller than the symbol size.
The octahedral tilting can be expressed in terms of three tilt angles: θ, φ and Φ (O'Keeffe and Hyde, 1977; Zhao et al., 1993), representing the tilt of the octahedra about the pseudo-cubic [110]pc, [001]pc and [111]pc directions of the aristotype Pm perovskite structure. These can be calculated from the a, b and c unit-cell lattice parameters of the Pbnm perovskite structure assuming rigid and non-distorted octahedra according to Zhao et al. (1993):
with the Φ tilt being a combination of Eqs. (1) and (2).
The θ tilt results from the two anti-phase tilts of equal magnitude about the [100]pc and [010]pc directions (Glazer, 1972; Megaw, 1973), and it is indicated as the out-of-phase tilt, whereas the φ tilt results from the tilt about the [001]pc direction and is indicated as the in-phase tilt. The θ and φ tilt angles are plotted as a function of Vmol in Fig. 3. The octahedral tilting increases from CaGeO3, for which both tilt angles are close to 3°, to CaPbO3, for which the tilt angles are larger than 10° with θ > φ. CaIrO3, CaRuO3 and CaRhO3 have a much more pronounced octahedral tilting, with the out-of-phase tilt having a larger deviation from the trend defined by the other CaB4+O3 perovskites (Fig. 3).
Table 1Unit-cell lattice parameters and polyhedral volumes of the CaIrO3 perovskite-type phase measured under ambient conditions and high pressure (Pbnm space group).
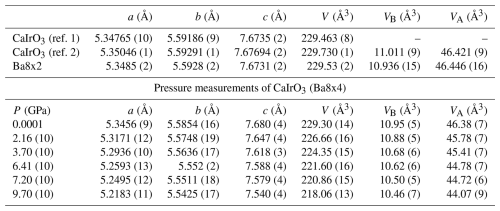
Ref 1: Boffa Ballaran et al. (2007). Ref. 2: Cheng et al. (2011).
Table 2Unit-cell lattice parameters and polyhedral volumes of CaB4+O3 perovskite-type phases reported in the literature (Pbnm space group).
Since the assumption of rigid octahedra is valid only as a first approximation and, moreover, tilting of the octahedral framework also induces a distortion of the A polyhedron, the atomic positions obtained in this study have been used to calculate the Irreps for the Pbnm CaIrO3 structure with respect to an ideal cubic perovskite having the same volume using the ISODISTORT programme (Campbell et al., 2006). The same calculations have been performed for the other CaB4+O3 perovskites. As expected, the largest amplitudes computed by the programme are those relative to the symmetry-adapted modes and , which are exclusively responsible for the octahedral tilting. From the amplitude of these two symmetry-adapted modes, it is therefore possible to calculate the out-of-phase and in-phase tilts independently of the polyhedral distortion using the equations reported by Wang and Angel (2011):
where d′ is derived from the output of ISODISTORT following the procedure outlined by Wang and Angel (2011) (see their example for MgSiO3 bridgmanite).
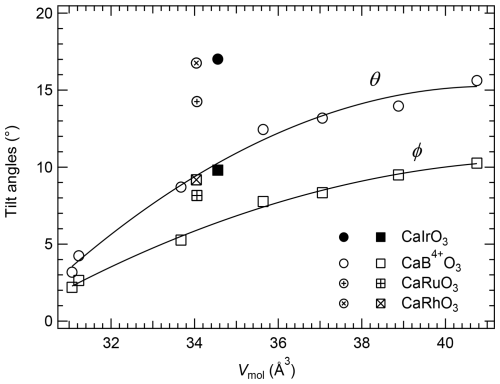
Figure 3Variation in the out-of-phase, θ (circles), and in-phase, φ (squares), tilts of orthorhombic CaB4+O3 pv as a function of their molar volumes. The tilts of CaIrO3 obtained in this study, as well as those of CaRuO3 and CaRhO3, clearly deviate from the trends. The solid curves are polynomial weighed fits through the CaB4+O3 perovskite data from Table 2, with CaRhO3 and CaRuO3 excluded. The uncertainties are smaller than the symbol size.
The variations with Vmol of and (Fig. 4) are different from the tilting patterns obtained using the unit-cell lattice parameter values (Fig. 3). Not only is their variation with the molar volume of the CaB4+O3 perovskites linear, but the values of the in-phase and out-of-phase tilts are also very similar for all analysed samples. However, similarly to what was observed for the tilts obtained from the unit-cell parameters, the values calculated for CaIrO3, CaRhO3 and CaRuO3 are much larger and deviate from the trend described by the other CaB4+O3 perovskites. The difference between the two sets of calculations is due to the fact that the polyhedral distortion in these perovskites is not negligible; nevertheless, even as just a first approximation, the tilt angles obtained from the unit-cell lattice parameters, based on the assumption of rigid and undistorted polyhedra, already highlight the distinctive behaviour of perovskites having a Pt-group element (i.e. Ir, Ru and Rh) occupying the octahedral site.
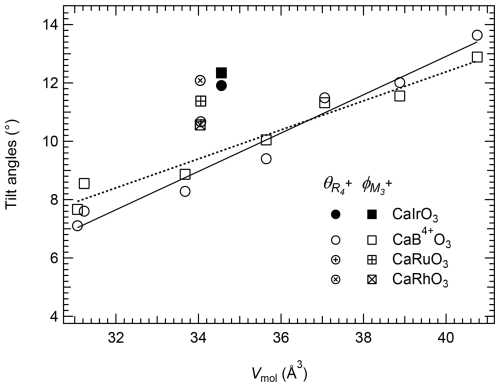
Figure 4Variation in the out-of-phase, , and in-phase, , tilts calculated from the symmetry-adapted and mode amplitudes for CaB4+O3 pv. Note that the values obtained for CaIrO3, CaRuO3 and CaRhO3 clearly deviate from the trends described by the other CaB4+O3 pv. The estimated uncertainties are smaller than the symbol size. The solid and dotted lines are linear weighed fits through the and CaB4+O3 perovskite data, respectively.
The polyhedral distortion is mainly due to the symmetry-adapted mode and, in particular, the displacement of the Ca atoms ( A) (Fig. 5), since the amplitudes of the symmetry-adapted modes and are practically negligible. The distortion of the A-site polyhedron increases due to the displacement of the Ca atoms toward one side of the cavity, probably in order to have better coordination with at least some of the oxygen atoms, as a consequence of the octahedral frameworks becoming larger with the increasing size of the cation occupying the B site. However, the A sites of CaIrO3, CaRuO3 and CaRhO3 perovskites appear much more distorted than expected from the size of the octahedral cations. The above observations, therefore, suggest that, in the case of the CaO orthorhombic perovskite structures, octahedral tilting and distortion result primarily from the mismatch of the B- and A-cation sizes, whereas, in the case of the Pt-group element perovskites, the B site appears larger than expected, giving rise to a larger orthorhombic distortion. This behaviour may be due to the small difference in electronegativity between the Pt-group elements and the oxygen, causing a weaker and therefore longer-than-expected ionic bond at the octahedral site.
CaIrO3, CaRuO3 and CaRhO3 perovskites transform under high pressure to a post-perovskite phase (orthorhombic Cmcm CaIrO3-type structure), which is either the stable phase under ambient pressure (CaIrO3) or the metastable phase that is easily quenchable (Hirose and Fujita, 2005; Kojitani et al., 2007a, b; Shirako et al., 2009). A Cmcm CaIrO3-type structure has also been found for CaPtO3 (Inaguma et al., 2008); however, no report of a perovskite structure of this compound is present in the literature.
Akaogi et al. (2010) have reported that there is an inverse correlation between the transition pressure of these perovskites and the tilt angle Φ (Eq. 3). CaSnO3 pv has a Φ angle similar to that of CaRuO3 pv, and it has been reported to transform to a post-perovskite structure (Tateno et al., 2010) that can be quenched under room conditions, albeit just as a mixture of perovskite and post-perovskite phases rather than a single phase as for the other three perovskites. The transition, however, occurs above 40 GPa, i.e. at pressures much larger than expected from the Φ tilt trend defined by the Pt-group perovskites (Akaogi et al., 2010), suggesting that not only octahedral tilting but also polyhedral distortion may play a significant role in determining the pressure at which the perovskite-to-post-perovskite phase transformation occurs. The large tilting and polyhedral distortion may also significantly affect the compressibility of these three perovskites, making them stiffer than expected, as observed for CaIrO3 pv (Boffa Ballaran et al., 2007).
3.2 Compression mechanism of CaB4+O3 perovskites
The unit-cell lattice parameters of the Ba8x4 crystal loaded in the DAC under room pressure before loading the pressure-transmitting medium were obtained from a total of 354 non-unique reflections and are in good agreement with those determined for the Ba8x2 crystal (Table 1). The bulk compression behaviour of the Ba8x4 crystal is also in agreement with the equation of state determined by Boffa Ballaran et al. (2007), confirming that CaIrO3 pv is much stiffer than other CaB4+O3 perovskites (Boffa Ballaran et al., 2007; Ross and Chaplin, 2003). The high-pressure structural evolution of non-cubic ABO3 perovskites under hydrostatic conditions depends on the relative compression of the BO6 octahedra and AO12 polyhedra (e.g. Zhao et al., 2004b), with consequent change in octahedral tilting. For A2+B4+O3 perovskites, the BO6 octahedra are expected to be less compressible than the AO12 polyhedra, and the octahedral tilting is therefore expected to increase with pressure. This is the case for CaSnO3 (Zhao et al., 2004a) and CaTiO3 (Zhao et al., 2011), for which it was shown that the ratio decreases with increasing pressure. In contrast, the ratio obtained in this study for CaIrO3 pv appears to be independent of pressure (Fig. 6). A weighed linear fit through the data even suggests a slight increase in the ratio with pressure, implying that the octahedral site of CaIrO3 pv has a compressibility similar to or even larger than that of the A site, in contrast to the observed trend for the other two CaB4+O3 perovskites.
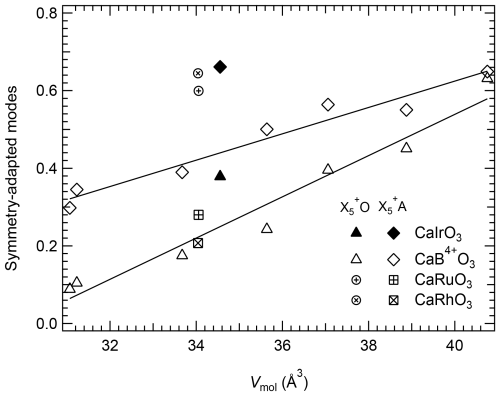
Figure 5Variation with molar volume in the amplitude of the symmetry-adapted modes relative to the displacement of the oxygen, O, and Ca atoms, A, from the ideal positions of the aristotype cubic structure for CaB4+O3 pv. The values obtained for CaIrO3, CaRhO3 and CaRuO3 deviate from the trends described by the other CaB4+O3 pv. Solid lines are linear weighed fits through the CaB4+O3 pv data.
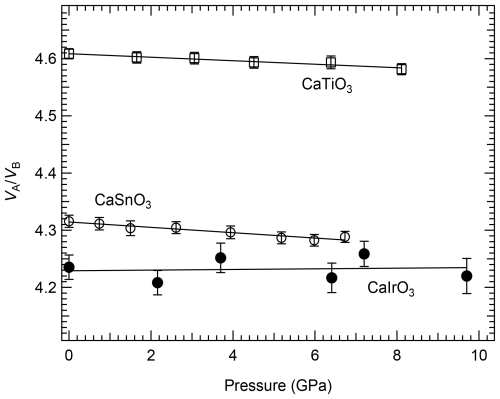
Figure 6Variation with pressure in the ratio of CaIrO3 pv (this study) compared with values for CaSnO3 (Zhao et al., 2004a) and CaTiO3 (Zhao et al., 2011). In contrast to what is expected for A2+B4+O3 perovskites, the ratio of CaIrO3 pv is independent of pressure, at least in the pressure range investigated in this study.
Since the ratio can be used to describe the octahedral tilting of orthorhombic perovskites, i.e. the larger the tilt, the larger the deviation of the ratio from the ideal value of 5 in the cubic aristotype perovskite, it appears that the orthorhombic distortion of CaIrO3 due to octahedral tilting remains constant at least up to 10 GPa, in contrast to the other two CaB4+O3 perovskites, whose tilting increases with pressure (Zhao et al., 2004a, 2011).
This behaviour is quite surprising since it has been suggested that the perovskite-to-post-perovskite transformation is driven by the repulsion between inter-octahedral anions, which become closer due to the increasing octahedral tilting with pressure. Martin and Parise (2008) analysed the structural data of 70 perovskites under ambient and high-temperature or high-pressure conditions and suggested that a minimum ratio of ∼ 4.03 should be reached in order for the inter-octahedral anion distances to be equal to the intra-octahedral anion distances, hence causing the transition to post-perovskite. These authors show that the ratio decreases and octahedral tilting increases with pressure not only for CaSnO3, but also for other perovskite-structured materials, for example NaMgF3 and SrCeO3. This is also valid for the end-member MgSiO3 bridgmanite and Al-bearing bridgmanite, for which it has been found that the A site is more compressible than the B site (Criniti et al., 2024). Following Martin and Parise (2008), the distances l[001] between the oxygens in the 8d Wyckoff position along the c axis of the CaB4+O3 perovskites, normalised with respect to the intra-oxygen distance of the accompanying octahedral site, O–Ooct, are reported as a function of the ratio for the CaB4+O3 perovskites (Fig. 7). The O–Ooct distances of CaTiO3 at 8.11 GPa of CaSnO3 at 6.73 GPa and CaIrO3 at 9.7 GPa are also reported in the same figure. All data points closely follow the mathematical model defined by Martin and Parise (2008), likely due to the fact that this inter-anion distance is not sensitive to deformation of the octahedra, as already noted by those authors.
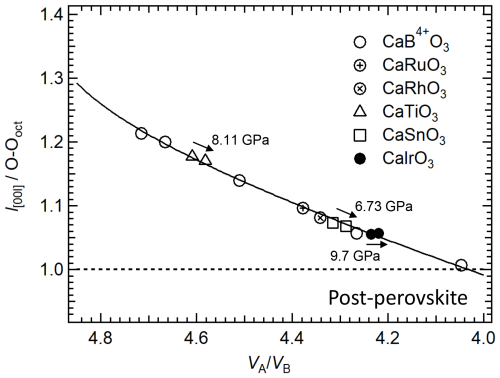
Figure 7Variation with the ratio in the inter-oxygen distances l[001] along the c axis, normalised with respect to the intra-oxygen distance of the accompanying octahedral site, O–Ooct, for the CaB4+O3 pv. The high-pressure values for CaIrO3 pv (this study), CaSnO3 (Zhao et al., 2004a) and CaTiO3 (Zhao et al., 2011) are also reported. The solid line is the mathematical model reported by Martin and Parise (2008).
There are, however, two important points to be made: (i) the O–Ooct values of CaRuO3 and CaRhO3 are larger than those of CaSnO3; therefore if tilting alone was driving the perovskite-to-post-perovskite transition, one would expect a higher transition pressure for CaRuO3 and CaRhO3 than for CaSnO3, contrary to what has been reported, and (ii) in spite of the smaller pressure range investigated for CaTiO3 and CaSnO3, the change in O–Ooct due to increasing pressure is much more accentuated for these two perovskites than for CaIrO3 (this study), confirming that the octahedral tilting for CaIrO3 does not change at least up to 10 GPa. This suggests that for CaIrO3, and likely also for the other Pt-group perovskite-structured materials, octahedral tilts alone are not sufficient to explain the mechanism for the perovskite-to-post-perovskite transition. A major factor driving the phase transformation appears to be the displacement of the Ca atoms, which is already quite large under room conditions for the Pt-group element perovskites (Fig. 5) and increases significantly with pressure for CaIrO3 relative to the displacement of Ca in the CaSnO3 structure (Fig. 8).
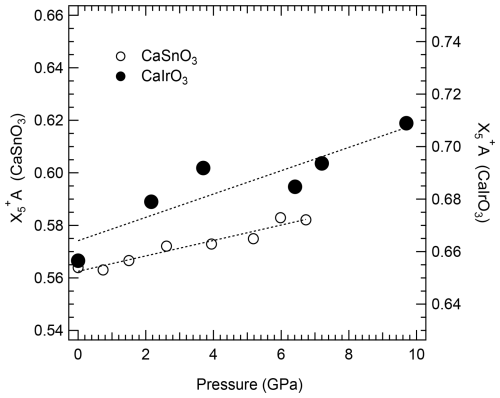
Figure 8Variation with pressure in the amplitude of the symmetry-adapted modes relative to the displacement of the Ca atoms, A, from the positions of the aristotype cubic structure for CaSnO3 (left axis) and CaIrO3 pv (right axis). The scale of the two axes is identical to better visualise the larger increase in the distortion of CaIrO3 compared to CaSnO3. The dotted lines are guides to eye. Uncertainties are smaller than the symbol sizes.
It is very likely that, as the Ca atoms are displaced toward one side of the large A site in CaIrO3 pv, some of the Ca–O bond distances become shorter, giving rise to a much stiffer A site with respect to that of the CaSnO3 structure. Because the octahedral tilting in CaIrO3 is hindered by such polyhedral distortion, we can expect that the structure becomes more unstable under compression, favouring the transformation to the post-perovskite phase at pressures lower than would be expected if octahedral tilting alone were the main cause of the transition.
Refinements to single-crystal intensity data collected under both ambient and high-pressure conditions have allowed us to clarify the tilting and distortion of orthorhombic CaIrO3 pv. Both octahedral tilting (Figs. 3 and 4) and A-site distortion (Fig. 5) of this perovskite are much larger with respect to other CaB4+O3 perovskites, causing the CaIrO3 structure to be much stiffer than expected. The larger tilting and distortion appear to be characteristic of orthorhombic perovskites with a Pt-group element (i.e. Ir, Ru and Rh) occupying the octahedral site. Although there are no compressibility data for CaRuO3 and CaRhO3, it can be expected that the bulk modulus will also be larger than the trend shown by several other CaB4+O3 perovskites (Ross and Chaplin, 2003) for these two perovskites. Another peculiarity of the CaIrO3 structure is represented by the fact that the B and A sites have similar compressibility, at least up to 10 GPa, resulting in the octahedral tilt remaining constant instead of increasing with increasing pressure. The similar compression of the two sites may hinder oxygen–oxygen repulsion, which is the main factor responsible for stiffening the perovskite structure with increasing pressure, giving rise to a bulk modulus first pressure derivative, K′, smaller than 4. Ultimately, we can hypothesise that the relatively large stiffness of CaIrO3, the similar compressibility of the B and A sites, and the large distortion of the A-site polyhedron play an important role in determining the transformation pressure at which the perovskite structure transforms to the orthorhombic Cmcm post-perovskite and therefore should be a common feature of the Pt-group perovskites for which the same transformation has been reported (Akaogi et al., 2010).
All of the crystallographic data have been made available in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/ejm-37-699-2025-supplement.
TBB helped with the selection of the single crystals of CaIrO3 pv, analysed the data and wrote the first draft of the paper. KK prepared the diamond anvil for the high-pressure study, collected all single-crystal diffraction data and contributed to the final version of the paper. RGT synthesised and characterised the high-quality single crystals of CaIrO3 pv and contributed to the final version of the paper.
At least one of the (co-)authors is a member of the editorial board of European Journal of Mineralogy. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Celebrating the outstanding contribution of Paola Bonazzi to mineralogy”. It is not associated with a conference.
The authors thank Alexander Kurnosov for help with the diamond anvil cell loading. We also thank an anonymous reviewer, M. Akaogi and Wilson Crichton in particular for their useful suggestions.
The Centre for Planetary Habitability is funded by CoE grant no. 332523 from the Research Council of Norway. This research has been supported by the Deutsche Forschungsgemeinschaft (grant no. BO2550/4-2).
This paper was edited by Luca Bindi and reviewed by M. Akaogi, Wilson Crichton, and one anonymous referee.
Akaogi, M., Shirako, Y., Kojitani, H., Takamori, S., Yamaura, K., and Takayama-Muromachi, E.: Post-perovskite transitions in CaB4+O3 at high pressure, J. Phys. Conf. Ser., 215, 012095, https://doi.org/10.1088/1742-6596/215/1/012095, 2010.
Angel, R. J., Downs, R. T., and Finger, L. W.: High-temperature-high-pressure diffractometry, in: High-Temperature and High-Pressure Crystal Chemistry, edited by: Hazen, R. M. and Downs, R. T., 41, 559–596, Reviews in Mineralogy, Mineralogical Society of America, Chantilly, Virginia, ISBN 0-939950-53-7, 2000.
Angel, R. J.: Absorption corrections for diamond-anvil pressure cells implemented in the software package Absorb6.0, J. Appl. Cryst., 37, 486–492, https://doi.org/10.1107/S0021889804005229, 2004.
Bensch, W., Schmalle, H. W., and Reller, A.: Structure and thermochemical reactivity of CaRuO3 and SrRuO3, Solid State lonics, 43, 171–177, https://https://doi.org/10.1016/0167-2738(90)90481-6, 1990.
Boffa Ballaran, T., Trønnes, R. G., and Frost, D. J.: Equations of state of CaIrO3 perovskite and post-perovskite phases, Am. Mineral., 92, 1760–1763, https://doi.org/10.2138/am.2007.2715, 2007.
Božin, E. S., Sartbaeva, A., Zheng, H., Wells, S. A., Mitchell, J. F., Proffen, Th., Thorpe, M.F., and Billinge, S. J. L.: Structure of CaMnO3 in the range 10 K 550 K from neutron time-of-flight total scattering, J. Phys. Chem. Solid., 69, 2146–2150, https://doi.org/10.1016/j.jpcs.2008.03.029, 2008.
Campbell, B. J., Stokes, H. T., Tanner, D. E., and Hatch, D. M.: ISODISPLACE: A web-based tool for exploring structural distortions, J. Appl. Cryst., 39, 607–614, https://doi.org/10.1107/S0021889806014075, 2006.
Cheng, J.-G., Zhou, J.-S., Goodenough, J. B., Sui, Y., Ren, Y., and Suchomel, M. R.: High-pressure synthesis and physical properties of perovskite and post-perovskite Ca1−xSrxIrO3, Phys. Rev. B, 83, 064401, https://doi.org/10.1103/PhysRevB.83.064401, 2011.
Clark, R. C. and Reid, J. S.: The Analytical Calculation of Absorption in Multifaceted Crystals, Acta Cryst. A, 51, 887–897, https://doi.org/10.1107/S0108767395007367, 1995.
Criniti, G., Boffa Ballaran, T., Kurnosov, A., Liu, Z., Glazyrin, K., Merlini, M., Hanfland, M., and Frost, D. J.: Thermal Equation of State and Structural Evolution of Al-bearing Bridgmanite. J. Geophys. Res.-Sol. Ea., 129, e2023JB026879, https://https://doi.org/10.1029/2023JB026879, 2024
de la Calle, C., Alonso, J. A., García-Hernández, M., and Pomjakushin, V.: Neutron diffraction study and magnetotransport properties of stoichiometric CaMoO3 perovskite prepared by a soft-chemistry route, J. Solid State Chem., 179, 1636–1641, https://doi.org/10.1016/j.jssc.2006.02.022, 2006.
Finger, L. W. and King, H. E.: A revised method of operation of the single-crystal diamond cell and refinement of structure of NaCl at 32 kbar, Am. Mineral., 63, 337–342, 1978.
Glazer, A.: The classification of tilted octahedra in perovskites, Acta Cryst. B, 28, 3384–3392, https://doi.org/10.1107/S0567740872007976, 1972.
Hirose, K. and Fujita, Y.: Clapeyron slope of the post-perovskite phase transition in CaIrO3, Geophys. Res. Lett., 32, L13313, https://doi.org/10.1029/2005GL023219, 2005.
Howard, C. J. and Stokes, H. T.: Group-theoretical analysis of octahedral tilting in perovskites, Acta Cryst. B, 54, 782–789, https://doi.org/10.1107/S0108768198004200, 1998.
Howard, C. J. and Stokes, H. T.: Group-Theoretical Analysis of Octahedral Tilting in Perovskites, Erratum, Acta Cryst. B, 58, 565–565, https://https://doi.org/10.1107/S010876810200890X, 2002.
Huang, R., Boffa Ballaran, T., McCammon, C. A., Miyajima, N., and Frost, D. J.: The effect of Fe-Al substitution on the crystal structure of MgSiO3 bridgmanite, J. Geophys. Res.-Sol. Ea., 126, e2021JB021936, https://doi.org/10.1029/2021JB021936, 2021.
Hübschle, C. B., Sheldrick, G. M., and Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL, computer programs, J. Appl. Cryst., 44, 1281-1284, https://doi.org/10.1107/S0021889811043202, 2011.
Inaguma, Y., Hasumi, K.-I., Yoshida, M., Ohba, T., and Katsumata, T.: High-Pressure Synthesis, Structure, and Characterization of a Post-perovskite CaPtO3 with CaIrO3-Type Structure, Inorg. Chem., 47, 1868–1870, https://doi.org/10.1021/ic701851e, 2008.
Kantor, I., Prakapenka, V., Kantor, A., Dera, P., Kurnosov, A., Sinogeikin, S., Dubrovinskaia, N., and Dubrovinsky, L.: BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements, Rev. Sci. Instrum., 83, 125102, https://doi.org/10.1063/1.4768541, 2012.
King, H. and Finger, L.: Diffracted beam crystal centering and its application to high-pressure crystallography, J. Appl. Cryst., 12, 374–378, https://doi.org/10.1107/S0021889879012723, 1979.
Kojitani, H., Furukawa, A., and Akaogi, M.: Thermochemistry and high-pressure equilibria of the post-perovskite phase transition in CaIrO3, Am. Mineral., 92, 229–232, https://doi.org/10.2138/am.2007.2358, 2007a.
Kojitani, H., Shirako, Y., and Akaogi, M.: Post-perovskite phase transition in CaRuO3, Phys. Earth Plan. Int., 165, 127–134, https://doi.org/10.1016/j.pepi.2007.09.003, 2007b.
Kurnosov, A., Kantor, I., Boffa Ballaran, T., Lindhardt, S., Dubrovinsky, L., Kuznetsov, A., and Zehnder, B. H.: A novel gas-loading system for mechanically closing of various types of diamond anvil cells, Rev. Sci. Instrum., 79, 045110, https://doi.org/10.1063/1.2902506, 2008.
Levin, I., Amos, T. G., Bell, S. M., Farber, L., Vanderah, T. A., Roth, R. S., and Toby, B. H.: Phase equilibria, crystal structures, and dielectric anomaly in the BaZrO3-CaZrO3 system, J. Solid State Chem., 175, 170–181, https://doi.org/10.1016/S0022-4596(03)00220-2, 2003.
Marbouh, N., Belabbes, M., Khelfaoui, F., Khalfallah, B., Bentayeb, A., and Driss Khodja, M.: Computational study of the structural, elastic, electronic and thermoelectric properties of the orthorhombic perovskite CaIrO3 compound, Comput. Condens. Matter, 29, e00594, https://https://doi.org/10.1016/j.cocom.2021.e00594, 2021.
Martin, C. D. and Parise, J. B.: Structure constraints and instability leading to the post-perovskite phase transition of MgSiO3, Earth Planet. Sc. Lett., 265, 630–640, https://doi.org/10.1016/j.epsl.2007.11.001, 2008.
McDaniel, C. L. and Schneidler, S. J.: Phase relations in the CaO-IrO2-Ir system in air, J. Solid State Chem., 4, 275–280, https://doi.org/10.6028/jres.075A.019, 1972.
Megaw, H. D.: Crystal Structures-A Working Approach, Saunders, Philadelphia, ISBN-10 0721662609, 1973.
Miller, S. C. and Love, W. F.: Tables of Irreducible Representations of Space Groups and Co-representations of Magnetic Space Groups, Boulder, Colarado, Pruett, 1967.
Mitchell, R. H.: Perovskites: Modern and ancient, Almaz Press, ISBN 0-9689411-0-9, 2002.
Murakami, M., Hirose, K., Kawamura, K., Sata, N., and Ohishi, Y.: Postperovskite phase transition in MgSiO3, Science, 304, 855–858, https://doi.org/10.1126/science.1095932, 2004.
Oganov, A. R. and Ono, S.: Theoretical and experimental evidence for a postperovskite phase of MgSiO3 in Earth's D′′ layer, Nature, 430, 445–448, https://doi.org/10.1038/nature02701, 2004.
O'Keeffe, M. and Hyde, B. G.: Some structures topologically related to cubic perovskite (E21), ReO3 (D09) and Cu3Au (L12), Acta Cryst. B, 33, 3802–3813, https://doi.org/10.1107/S0567740877012114, 1977.
O'Keeffe, M., Hyde, B. G., and Bovin, J.O.: Contribution to the crystal chemistry of orthorhombic perovskite: MgSiO3 and NaMgF3, Phys. Chem. Minerals, 4, 299–305, 1979.
Perez-Mato, J. M., Orobengoa, D., and Aroyo, M. I.: Mode crystallography of distorted structures, Acta Cryst. A, 66, 558–590, https://doi.org/10.1107/S0108767310016247, 2010.
Ross, N. L. and Chaplin, T. D.: Compressibility of CaZrO3 perovskite: Comparison with Ca-oxide perovskites, J. Solid State Chem., 172, 123–126, https://doi.org/10.1016/S0022-4596(02)00166-4, 2003.
Sasaki, S., Prewitt, C. T., and Liebermann, R. C.: The crystal structure of CaGeO3 perovskite and the crystal chemistry of the GdFeO3-type perovskites, Am. Mineral., 68, 1189–1198, 1983.
Sasaki, S., Prewitt, C. T., Bass, J. D., and Schulze, W. A.: Orthorhombic Perovskite CaTiO3 and CdTiO3: Structure and Space Group, Acta Cryst. C, 43, 1668–1674, 1987.
Shannon, R. D. and Prewitt, C. T.: Effective Ionic Radii in Oxides and Fluorides, Acta Cryst. B, 25, 925–946, https://doi.org/10.1107/S0567740869003220, 1969.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Cryst. C, 71, 3–8, https://doi.org/10.1107/S2053229614024218, 2015.
Shen, G., Wang, Y., Dewaele, A., Wu, C., Fratanduono, D. E., Eggert, J., Klotz, S., Dziubek, K. F., Loubeyre, P., Fat'yanov, O. V., Asimow, P. D., Mashimo, T., Wentzcovitch, R. M. M., and other members of the IPPS task group: Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020), High Press. Res., 40, 299–314, https://doi.org/10.1080/08957959.2020.1791107, 2020.
Shirako, Y., Kojitani, H., Akaogi, M., Yamaura, K., and Takayama-Muromachi, E.: High-pressure phase transitions of CaRhO3 perovskite, Phys. Chem. Minerals, 36, 455–462, https://doi.org/10.1007/s00269-009-0292-4, 2009.
Stølen, S. and Trønnes R. G.: The perovskite to post-perovskite transition in CaIrO3: Clapeyron slope and changes in bulk and shear moduli by density functional theory, Phys. Earth Planet. Int., 164, 50–62, https://doi.org/10.1016/j.pepi.2007.05.009, 2007.
Tateno, S., Hirose, K., Sata, N., and Ohishi, Y.: Structural distortion of CaSnO3 perovskite under pressure and the quenchable post-perovskite phase as a low-pressure analogue to MgSiO3, Phys. Earth Planet. Int., 181, 54–59, https://https://doi.org/10.1016/j.pepi.2010.03.003, 2010.
Trønnes, R. G., Frost, D. J., Boffa Ballaran, T., and Stølen, S.: The perovskite to post-perovskite transition in CaIrO3, American Geophysical Union Fall Meeting, MR11A97, 2006.
Wang, D. and Angel, R. J.: Octahedral tilts, symmetry-adapted displacive modes and polyhedral volume ratios in perovskite structures, Acta Cryst. B, 67, 302–314, https://doi.org/10.1107/S0108768111018313, 2011.
Yamamoto, A., Khasanova, N. R., Izumi, F., Wu, X.-J., Kamiyama, T., Torii, S., and Tajima, S.: Crystal Structure and Its Role in Electrical Properties of the Perovskite CaPbO3 Synthesized at High Pressure, Chem. Mater., 11, 747–753, https://doi.org/10.1021/cm980629p, 1999.
Zhao, J., Ross, N. L., and Angel, R.J.: Tilting and distortion of CaSnO3 perovskite to 7 GPa determined from single-crystal X-ray diffraction, Phys. Chem. Minerals, 31, 299–305, https://doi.org/10.1007/s00269-004-0391-1, 2004a.
Zhao, J., Ross, N. L., and Angel, R. J.: New view of the high-pressure behaviour of GdFeO3-type perovskites, Acta Cryst. B, 60, 263–271, https://doi.org/10.1107/S0108768104004276, 2004b.
Zhao, J., Ross, N. L., Wang, D., and Angel, R. J.: High-pressure crystal structure of elastically isotropic CaTiO3 perovskite under hydrostatic and non-hydrostatic conditions, J. Phys.: Condens. Matter, 23, 455401, https://doi.org/10.1088/0953-8984/23/45/455401, 2011.
Zhao, Y., Weidner, D. J., Parise, J. B., and Cox, D. E.: Thermal expansion and structural distortion of perovskite-Data for NaMgF3 perovskite, Part I, Phys. Earth Planet. Int., 76, 1–34, https://doi.org/10.1016/0031-9201(93)90051-A, 1993.



