the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Chrysoberyl from the Sabatini Volcanic Complex (Latium, Italy): chemical and petrological peculiarities
Giuseppe Illuminati
Silvia Musetti
Fabio Bellatreccia
Cristian Biagioni
Enrico Caprilli
Ahmad Rabiee
Marco E. Ciriotti
This paper describes mineralogical, chemical, and petrological features of an iron-rich chrysoberyl, containing up to 0.31 Fe atoms per formula unit (up to 17.24 wt % as Fe2O3), found in some rare volcanic ejecta of the Sabatini Volcanic Complex in central Italy. These rocks, locally named “sanidinites”, are ultrapotassic and undersaturated in silica, and they are well known for their abundance of HFSE-LILE-REE-rich well-crystallized minerals (HFSE: high‐field-strength element; LILE: large‐ion lithophile element; REE: rare earth element). Chrysoberyl occurs as yellow to brown crystals up to 2 mm in size, typically twinned, sometimes with penetration trilling of pseudohexagonal habit. Refined unit-cell parameters are a=9.4507(5) Å, b=5.4975(3) Å, c=4.4364(2) Å, and V=230.49(2) Å3. Its crystal structure has been refined the in Pnma space group down to R1=0.0397 for 420 unique reflections with F>4σ(F) and 41 refined parameters. The study of this occurrence provides insight into the partitioning of Fe into the Al(2) site and the role of Fe-to-Al substitution in the anisotropic chemical expansion of the structure. The differences between this occurrence and those reported in the literature are discussed, proposing a new metasomatic genesis for this anomalous chrysoberyl occurrence to explain the observed textural, chemical, and mineralogical evidence.
- Article
(11488 KB) - Full-text XML
-
Supplement
(395 KB) - BibTeX
- EndNote
Many studies have been devoted to fully characterizing chrysoberyl (BeAl2O4) and its geological occurrence. This interest is motivated by the fact that this mineral is, with beryl [Be3Al2(Si6O18)], bertrandite [Be4(Si2O7)(OH)2], and phenakite [Be2(SiO4)], one of the most widespread hosts for beryllium, now recognized as a critical raw material (European Commission, 2023). Additionally, synthetic analogues of chrysoberyl are used in industrial, medical, and military applications as gain media for tunable solid-state lasers, valued for their low symmetry and strong birefringence (Landthaler and Hohenleutner, 2006; Li et al., 2008; Bouzari et al., 2004; Šulc and Jelínková, 2013; Fibrich et al., 2017). Finally, chrysoberyl and its Cr-rich variety, alexandrite, are well known for their gemmological significance (Gao et al., 2023). For these reasons, much effort was put into both understanding crystallization in order to synthesize the sought-after metal-doped chrysoberyl crystals (Bukin et al., 1981; Gromalova et al., 2012; Malsy and Armbruster, 2012; Schmetzer et al., 2012) and identifying and characterizing the chrysoberyl-rich deposits (YinCe et al., 2022).
As reported in Sun et al. (2019) and YinCe et al. (2022), more than 380 chrysoberyl deposits are reported all around the world: mainly in Pakistan, China, India, Myanmar, and Sri Lanka (Asia); in the United States, Brazil, and El Salvador (Americas); in Zimbabwe, Tanzania, and Madagascar (Africa); and in Bulgaria, Czech Republic, Norway, Poland, Spain, and Russia (Europe). Some occurrences are also known in Italy, where chrysoberyl is reported as a rare accessory mineral in Alpine granitic pegmatites (e.g. Vignola et al., 2018).
The genesis of chrysoberyl in these deposits, most of which are rare-metal pegmatites and sometimes metamorphosed, has been well documented and extensively studied. The stability fields and forming reaction are well established, and the geochemical and structural properties of chrysoberyl are relatively homogeneous (Beus, 1966; Franz and Morteani, 1984; Černý et al., 1992; London and Evensen, 2002).
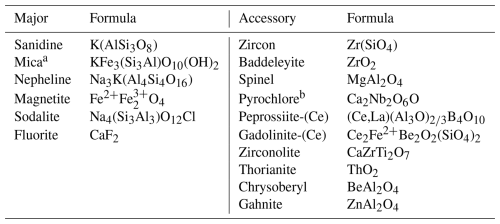
In this paper, we report the first occurrence of chrysoberyl (BeAl2O4) in rare volcanic ejecta from the Sabatini Volcanic Complex in central Italy. These volcanic ejecta, often locally called “sanidinites” because of the abundance of sanidine, are ultrapotassic and silica-undersaturated rocks. They are found within pyroclastic deposits associated with Plio-Quaternary volcanism in central-southern Italy and are well known for the abundance of HFSE-LILE-REE1 minerals, and in particular several Be minerals are observed (Table 1). Chrysoberyl represents the first Be-oxide reported for these rocks.
This new occurrence of chrysoberyl has some unusual features. Indeed, the absence of typically associated minerals, in particular the total absence of quartz that “is always present together with chrysoberyl in nature” (Franz and Morteani, 1984), along with the chemical and structural peculiarities may indicate a different genetic process. In this work, we provide a chemical, spectroscopic, optical, and structural characterization of this new chrysoberyl occurrence, comparing it with other reported occurrences and making a hypothesis about the formation environments.
2.1 Geological setting and studied sample description
The Latium volcanic province is a portion of the Roman Comagmatic Region (hereinafter referred to as RR; Washington, 1906) and comprises four main volcanic complexes, which developed along a NNW–SSE-oriented zone, parallel to the Tyrrhenian margin of central Italy. It originated from the extensional dynamics related to the opening of the Tyrrhenian back-arc basin, with the consequent crustal thinning, accompanied by normal faults along which the alkaline magma was conveyed. The pre-volcanic substrate crossed by these intrusions is fundamentally composed by the pre-orogenic Tuscan (siliciclastic sandstone, limestones, marls, and other sedimentary rocks), Ligurian (sedimentary, metamorphic, and ophiolitic rocks), and Sabina/Umbria/Marche or Lazio/Abruzzi successions (essentially limestones and sedimentary carbonate rocks), as well as by the more recent post-orogenic Quaternary marine and fluvial siliciclastic deposits (Peccerillo, 2005, 2016). The Latium volcanic province is bordered to the north with older Tuscany volcanic rocks (in structural continuity) and to the south with an important tectonic structure named the “Ancona–Anzio line” or “Olevano–Antrodoco line”. These volcanic complexes are nowadays easily recognized by their respective collapsed calderas and volcano-tectonic depressions, which now mostly constitute lake basins. From northwest to southeast, we can follow chronologically their volcanic activity: the Vulsini complex, where volcanic activity was between 0.6 to 0.15 Ma; the Vico complex, active from 0.42 to 0.1 Ma; the Sabatini district, active through several eruptive centres from 0.8 to 0.04 Ma (De Rita et al., 1983, 1993, 1997; Di Filippo, 1993); and the Colli Albani volcanic complex (Alban Hills), active between 0.6 to 0.02 Ma. Samples containing chrysoberyl were collected near Trevignano Romano village (Rome) in the Sabatini Volcanic Complex (Funiciello et al., 1988), within a pyroclastic deposit (Fig. 1) associated with the phreatomagmatic activity of the San Bernardino maar. The volcanic activity has been dated, through the method, to 0.170(5) Ma (Sottili et al., 2010).
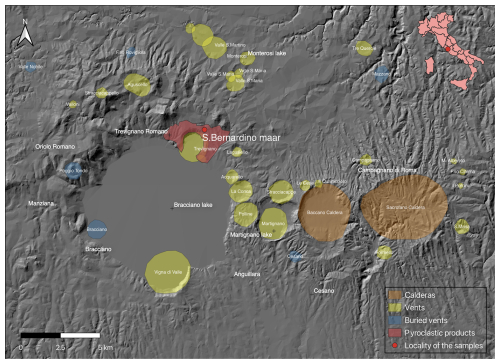
Figure 1Map of the Sabatini Volcanic Complex where main volcanic vents and calderas are reported: the red point represents the area where samples were collected. DEM was obtained from Tinitaly (CC BY 4.0 license; Tarquini et al., 2023).
RR volcanic rocks are mostly ultrapotassic and silica-undersaturated (Conticelli and Peccerillo, 1992; Peccerillo, 2016), where alkali-syenites and feldspathoid-rich syenites represent the most frequent, although rare, volcanic ejecta. These ejecta are well known for abundance of Li-, Be-, B-, Zr-, Nb-, Th-, U-, and REE-bearing minerals and for the presence of feldspathoids like nepheline, leucite, and rare (sometimes unique) cancrinite-group minerals. This mineral assemblage represents the petrological features of chrysoberyl, although each ejecta sample exhibits different mineralogical characteristics.
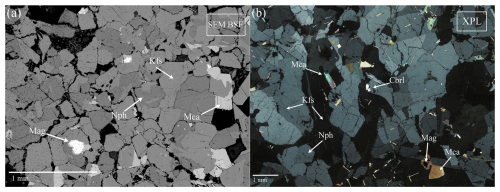
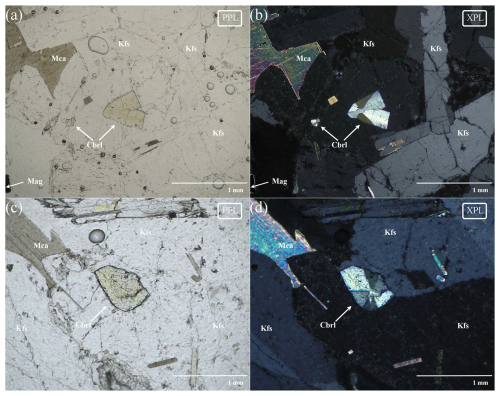
Figure 2(a) SEM backscattered electron (BSE) micrograph; (b) optical micrograph with cross-polarized light (XPL). Mca: mica, Kfs: K-feldspar, Mag: magnetite, Nph: nepheline, Cbrl: chrysoberyl (Warr, 2021).

Figure 3(a) Brown chrysoberyl with typical pseudohexagonal twinning of about 1.5 mm on sanidine. Chrysoberyl is surrounded on the top and on the left by two dark iron-rich mica stacked crystals (field of view is 5.5 mm); (b) green gahnite group 0.3 mm wide formed by tiny deformed octahedral crystals (field of view is 0.9 mm). In this sample, gahnite typically occurs as inclusions in hexagonal, colourless nepheline crystals within the miarolitic cavities.
Among the collected ejecta, chrysoberyl has been identified in six different sanidinites (samples C1 to C6) where it occurs as a rare accessory mineral. These ejecta are typically rounded holocrystalline rocks of about 10–20 cm in diameter, mostly equigranular with small miarolitic cavities (up to 5 mm in diameter), composed mainly of alkali feldspar (sanidine), nepheline, minor sodalite, magnetite, and brown mica (Fig. 2). Chrysoberyl occurs in the cavities within K-feldspar phenocrysts as crystals of yellow to brown colour, forming groups of twinned tabular crystals with characteristic V shape, sometimes with the typical penetration trilling of pseudohexagonal habits, not exceeding 2 mm in diameter (Fig. 3a). Typically, the associated minerals are Zr-Nb-Th-U-REE phases (zircon, peprossiite-(Ce), gadolinite-(Ce), baddeleyite, pyrochlore, zirconolite; see Table 2). Moreover, chrysoberyl is frequently associated with gahnite (Fig. 3b), a very peculiar association already reported for the chrysoberyl occurrences in Western Australia (Downes and Bevan, 2002) and western Spain (Merino et al., 2013).
2.2 Chemical analysis
Thin sections were prepared for samples from C1 to C5 (C6 chrysoberyl was provided by the mineral collector Ezio Curti, and no thin section was made due to the scarcity of material). One chrysoberyl crystal for each sample (from C1 to C6) was embedded into a resin mount for chemical analysis. Images and chemical analyses were obtained at the laboratory of scanning electron microscopy and ultra/high-resolution microanalysis CERTEMA (Grosseto, Italy), with a field emission scanning electron microscope (FSEM), Merlin Zeiss II, equipped with a GEMINI II optical column, which performs an automatic commutation between the cross beam (analytic mode) and parallel beam (used for high resolution). The FSEM is equipped with four detectors: two in-lens detectors for secondary electron (SE) and energy selective backscatter (EsB) for high-angle backscattered electrons (BSE) and two in-chamber detectors for both SE and BSE (AsB, angle selective backscatter). Quantitative chemical microanalyses were performed by combining energy-dispersive X-ray spectroscopy (EDS) (Oxford Instruments X-Max 50 mm2 spectrometer) and wavelength-dispersive X-ray spectroscopy (WDS) (Oxford Instruments INCA WAVE 700 spectrometer) to optimize resolution and sensitivity of measurements. BSE images and EDS-WDS spectra were recorded using a 15 kV electron beam acceleration at 8.5 mm working distance and a beam size of about 800 nm. Current was measured before each analysis at about 2.7 nA with the use of a Faraday cup connected to a picoammeter. The standards, elements, and analytical lines used for WDS quantification were respectively the following: (1–2) ilmenite, Fe and Ti, Kα; (3) franklinite, Al, Kα; (4) gahnite, Zn, Kα; (5) anorthite, Si and Ca, Kα; (6) strontianite, Sr, Lα; and (7) anorthoclase, Na, Kα. The background was taken to the left and right of the WDS line. Standards used for EDS quantification (Kα) were (a) anorthoclase, Si; (b) diopside, Ca; (c) albite, Na; (d) microcline, K; (e) magnetite, Fe; (f) ilmenite, Ti; (g) gahnite, Zn; (h) MgO, Mg; and (i) manganosite, Mn. The Ga contents were estimated from the Lα line in standardless modality. Acquisition times were 60 to 100 s on peak and 5 to 45 s for background: times were chosen to maintain analytical wt % error below 1 % respectively for major and trace elements.
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) analyses on chrysoberyl grains were performed at the Dipartimento di Scienze, Università Roma Tre, using a Teledyne photon machine Analyte Excite (193 nm) + ArF excimer laser ablation system coupled to a Thermo Scientific iCAP RQ (single quadrupole). All analyses were carried out at low oxide production rates (232Th < 0.5 %). Helium carrier gas streams of ∼0.5 and 0.3 L min−1 are set for the cell and the cup, respectively. Laser spot sizes of ∼35 and ∼20 µm (circles' diameters) were used for glass standards and unknown samples, respectively, with a 7 Hz pulse repetition rate and an energy density of 4 J cm−2. The ablation time was set at 65 s; it comprises a background acquisition lasting 15 s, followed by a sample ablation period of 25 s and then by a 25 s washout phase. Details regarding the instrumentation are provided in Rabiee et al. (2024).
The dwell times are 2–3 ms for the major elements' masses and 4 ms for the rest. Synthetic glass NIST-SRM-612 and NIST-SRM-610 reference materials (Jochum et al., 2011) were used to bracket analyses of unknowns, both used as external calibration, drift correction, and quality control. Data reduction was done using Iolite 4 software (Paton et al., 2011). The stoichiometric Be, Al, and Fe oxide contents of 100 wt % in chrysoberyl were used as the internal standard for LA-ICP-MS calibration. Limit of detection (LOD) data for measured elements are made available as in the Supplement.
2.3 Optical analysis
Among the samples, the C1 ejecta sample produced the best-quality chrysoberyl crystals, on which optical, diffraction, and IR spectroscopic analyses were performed. One of these crystals was mounted on a glass fibre and positioned on a spindle stage to determine the optical 2V angle from the measured extinction angles, using the EXCELIBR Excel spreadsheet (Steven and Gunter, 2017). The average refractive index with the Gladstone–Dale relation was also calculated (Mandarino, 1976, 1979) from unit-cell volume from single-crystal X-ray diffraction and Fe and Al contents from refined site occupancy.
2.4 X-ray diffraction
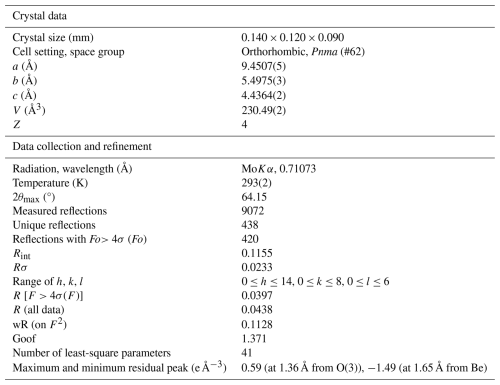
The chrysoberyl for X-ray diffraction was selected between the crystals from the C1 ejecta sample. Intensity data were collected using a Bruker D8 Venture diffractometer equipped with an air-cooled Photon III CCD detector and microfocus MoKα radiation (Centro per l'Integrazione della Strumentazione scientifica dell'Università di Pisa, CISUP, University of Pisa, Pisa, Italy). The detector-to-crystal distance was 38 mm. Data were collected using ω and φ scan modes, in 0.5° slices, with an exposure time of 10 s per frame. A total of 1682 frames was collected. The frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data were corrected for Lorentz polarization, absorption, and background using the Apex4 software package (Bruker AXS Inc., 2022). Unit-cell parameters were refined on the basis of the XYZ centroid of 4806 reflections above 20 σ(I) with 7.415° < 2θ<64.124°.
The statistical tests on the distribution of values () agree with the presence of a centre of symmetry, and the systematic absences support the space group Pnma. The crystal structure of chrysoberyl was refined using Shelxl-2018 (Sheldrick, 2015), starting from the atomic coordinates of Farrell et al. (1963). Neutral scattering curves, taken from the International Tables for Crystallography (Welberry, 2021), were used. An isotropic model converged to R1=0.0577, thus confirming the space group model. The displacement parameter at the Al(2) site was too small, suggesting the occurrence of a heavier atom replacing Al. Taking into account the results of electron microprobe analysis, the site occupancy at the Al(2) site was modelled using the scattering curves of Al vs. Fe, lowering R1 to 0.0442. After several cycles of anisotropic refinement for all atom positions, the R1 factor converged to 0.0397 for 420 unique reflections with F>4σ(F) and 41 refined parameters. Details of the data collection and crystal structure refinement are given in Table 3. Atom coordinates and equivalent isotropic displacement parameters are reported in Table 4, whereas Table 5 gives selected bond distances. Bond-valence calculation, shown in Table 6, was performed using the bond parameters of Gagné and Hawthorne (2015). The Crystallographic Information File (CIF) is made available in the Supplement.
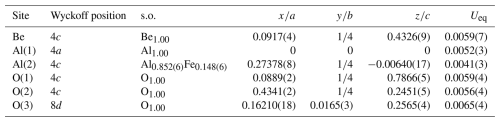
Table 4Sites, Wyckoff positions, site occupancy (s.o.), fractional atom coordinates, and equivalent isotropic displacement parameters (in Å2) for Fe-bearing chrysoberyl.
2.5 Infrared spectroscopy
Single-crystal micro-FTIR unpolarized spectra were collected on crystals from C1 ejecta due to their optical clarity and apparent absence of inclusions. The spectra were collected at LASR3 – Laboratorio Analisi di Superfici, Dipartimento di Scienze, Università Roma Tre, with a Thermo Scientific iS50 FTIR optical bench equipped with a Polaris source, coupled with a Nicolet Continuum FTIR microscope. The microscope is equipped with a liquid-nitrogen-cooled mercury–cadmium–telluride (MCT) detector and a Reflachromat objective (15×, numerical aperture 0.58). The spectra were obtained in transmission mode in the range 650–7000 cm−1, with a resolution of 4 cm−1, and 120 scans were averaged for each spectrum and background. Background spectra were collected on a CaF2 pellet over an area 100×100 µm2 wide. Sample detection area was equally wide. Data were collected and handled by Thermo Scientific OMNIC Atlµs software.
3.1 Petrological analysis
From petrographic and electron microscopy studies, along with chemical analyses (Tables 7 and 8), the chrysoberyl-bearing rock samples exhibit a rather homogeneous composition (Fig. 4), being primarily composed of sanidine as major component and many other feldspathoids as sodalite and nepheline. Quartz is absent in all studied samples, as typical for these undersaturated rocks where, in general, it was rarely observed. Moreover, an amorphous aluminosilicate phase, mostly occurring near miarolitic cavities like those hosting chrysoberyl, was observed.
Dark mica usually occurs shredded with typical high interference colours with cross-polarized light; the core of larger mica crystals is often replaced by magnetite and other tiny (Ti-Fe)-rich oxide inclusions presumably due to the dissociation of the iron-rich member of the trioctahedral biotite subgroup (Cygan et al., 1996).
Several minor phases were observed using SEM, including HFSE-REE minerals (mainly silicates, oxides, and phosphates). Among these minor phases, it is worth highlighting the presence of gahnite (ZnAl2O4) in aggregates of sub-micrometric crystals enclosed within chrysoberyl crystals and, less frequently, within nepheline and feldspars. This observation is of particular interest since the chrysoberyl–gahnite association was already reported in other geological contexts (Downes and Bevan, 2002; Merino et al., 2010, 2013).
3.2 Optical properties
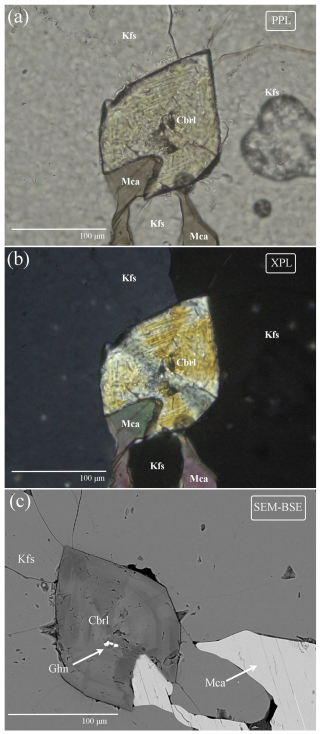
All the crystals of Trevignano chrysoberyl show a distinct optical zoning. In Fig. 5a (under plane-polarized light, PPL) a concentric zoning is shown, with distinct pleochroism of green (γ=b) to light yellow (β=a) in Fe-rich zones (also evident in Fig. 4c). Pleochroism is typical for transition-metal-rich varieties of chrysoberyl, such as alexandrite (the Cr-bearing variety). In this case, given the absence of Cr, the pleochroism is entirely due to the iron content and is, in fact, less pronounced in Fe-poor zones. Figure 5b shows the same crystal under cross-polarized light (XPL), where the crystal's twinning and oscillatory zonation were evident, as seen in the SEM-BSE micrograph presented in Fig. 5c. The 2V angle is 78.5° (with statistical p value = 0.673), with a calculated average refractive index of 1.838 (Mandarino, 1976, 1979). These values are higher than typical Fe-poor chrysoberyl, for which 2V value is about 70°, and n=1.746–1.756 (Anthony et al., 2003).
Table 9Representative chemical composition of analysed chrysoberyl crystals.
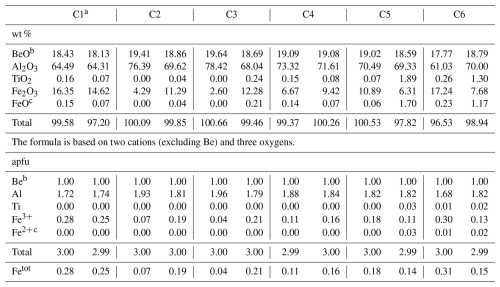
a From the same crystal studied by XRD. b From stoichiometry. c Fe2+ apfu considered equivalent to Ti4+ for electroneutrality.

Figure 6Variation of Fetot+ Ti with the Al (atoms per formula unit, apfu) in the studied chrysoberyl crystals. All data points align closely with the dashed line (), indicating Fe Al3+ and/or (Fe Ti4+) = 2Al3+ substitutions. For each sample (from C1 to C6), analyses are plotted showing a large field of values from 0.04 to 0.31 apfu of total substitution.
3.3 Chrysoberyl chemical composition
Representative SEM-WDS chemical analyses of Trevignano chrysoberyl are presented in Table 9. The most notable characteristic is the exceptionally high Fe content, making this mineral the Fe-richest chrysoberyl ever documented. Moreover, as far as we know, it is the Ti-richest natural occurrence, with Ti content up to 1.89 wt % as TiO2 (0.03 apfu, atoms per formula unit). As shown in Fig. 6, there is a clear negative linear correlation between Al and (Fe + Ti), attributed to two possible exchange mechanisms: Fe Al3+ and (Fe Ti4+)=2Al3+ (Fig. 5). The Fe2O3 content varies widely, ranging from 2.60 to 17.24 wt % (up to 0.31 Fetot apfu; for a comparison, see Žáček and Vràna, 2002).
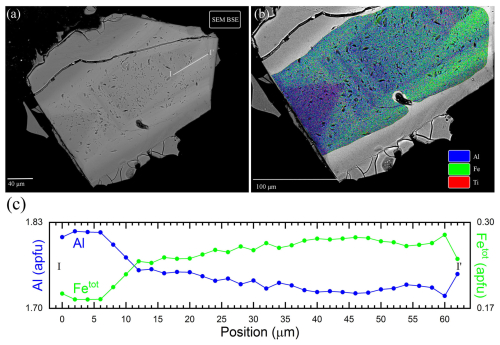
Figure 7(a) SEM-BSE chrysoberyl micrograph: lighter zones illustrating Fe-rich domains and darker zones showing Fe-depleted (or equivalently Al-rich) domains. The white line corresponds to the transect analysed in Fig. 8; (b) false colour SEM image with different colour intensities based on SEM-EDS count for Al (blue), Fe (green), and Ti (red); (c) Al and Fetot apfu measured along the I–I′ line presented in Fig. 7a, showing the typical oscillatory micro-zoning of the crystal.
Generally, the studied chrysoberyl crystals exhibit strong chemical zonation with sub-micrometric variations of Fe, Al, and Ti contents (Table 9). In particular, BSE images (Fig. 7a) reveal oscillatory zoning, with darker bands corresponding to Al-rich zones and brighter bands to Fe-rich zones, as can be seen from X-ray fluorescence maps and a microprobe traverse (Fig. 7b and c).
Table 10Minimum, maximum, and average elemental composition determined by LA-ICP-MS, compared to reference values.
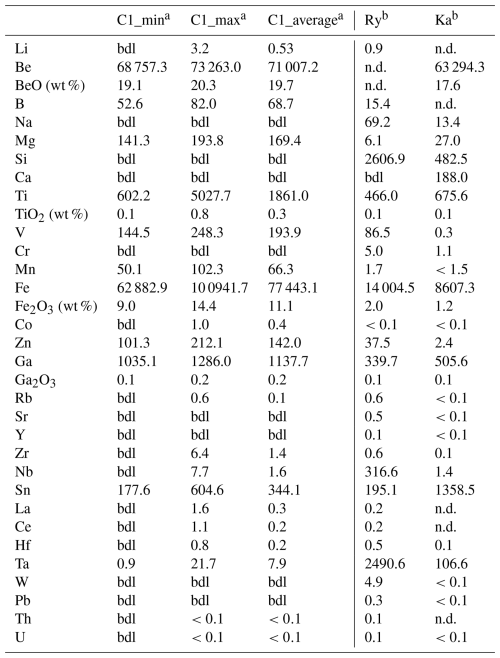
Ry: Rybnikova et al. (2023); Ka: Kanouo et al. (2016); a average, minimum, and maximum over six data points; b average of reported trace analyses; n.d.: not detected, bdl: below detection limit. Unless otherwise noted, concentrations are expressed in µg g−1.
LA-ICP-MS analyses (Table 10) confirm the exceptionally high content of Fe2O3 (up to 14.4 wt %), TiO2 (up to 0.8 wt %), and Ga2O3 (up to 0.2 wt %). However, the spot size of 20–35 µm could not reach the same spatial resolution of SEM, and these data typically represent a bulk analysis of both (Fe/Ti/Ga)-rich and (Fe/Ti/Ga)-poor zones. Contents of BeO from LA-ICP-MS (average 19.7 wt %) are in line with the stoichiometric one derived from calculated formula. Trevignano chrysoberyl also shows high V (248.3 µg g−1), Zn (212.1 µg g−1), Mg (193.8 µg g−1), and Mn (102.3 µg g−1) as well as low Ta (21.7 µg g−1) and Cr, always below detection limit. Detailed analytical results for all samples are provided in the Supplement.
3.4 X-ray diffraction and description of the structure
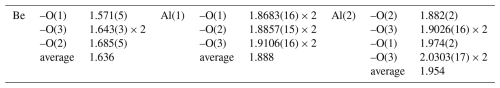
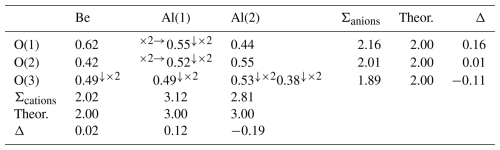
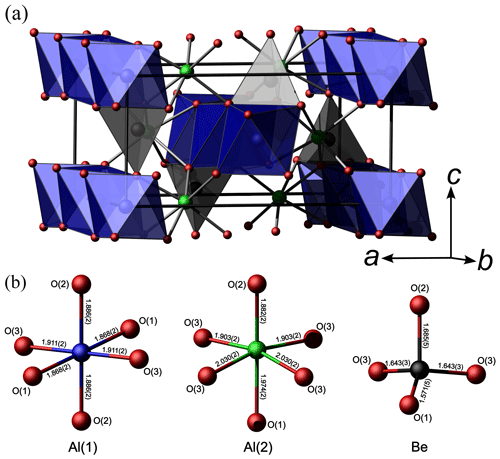
The chrysoberyl structure is isotypic with members of the forsterite-fayalite series. Its crystal structure (Fig. 8) can be described as a hexagonal close-packing of O atoms with Al occupying one-half of the octahedral interstices and with Be hosted at one-eighth of the tetrahedral cavities (Farrell et al., 1963). Aluminium occurs at the two symmetry-independent sites, namely Al(1) and Al(2). The former, at the Wyckoff position 4a, site symmetry −1, is fully occupied by Al3+, with bond distances ranging between 1.868 and 1.911 Å, with an average 〈Al–O〉 distance of 1.888 Å. On the contrary, Al(2), at Wyckoff position 4c, with site symmetry m, has a mixed (Al,Fe) occupancy, with refined site occupancy (Al0.85Fe). The replacement of Al by Fe is also supported by an increase in the average bond distance at this site, i.e. 1.954 Å. Bond distances range between 1.882 and 2.030 Å. The Al(2) site is more distorted than Al(1), as shown, for instance, by the larger Δd value (where Δd is defined as the difference between the longest and shortest distance within the octahedron), i.e. 0.148 and 0.043 Å, respectively. Bond-valence sums at Al(1) and Al(2) are 3.12 and 2.82 valence units (v.u.), in good agreement with the occurrence of trivalent cations. Beryllium is hosted at the Be site (Wyckoff site 4c, site symmetry m). It displays a tetrahedral coordination, with distances ranging from 1.571 to 1.685 Å, with an average value of 1.636 Å. Its bond-valence sum is 2.02 v.u., and no deviation from the full occupancy by Be is observed at this site. Oxygen anions are hosted at three independent positions, namely O(1), O(2), and O(3). Their bond-valence sums, ranging from 1.89 to 2.16 v.u., are in accordance with the presence of O2−. The structural formula of the studied sample is BeBeAl(1)AlAl(2)(Al0.85Fe)O4 (Z=4).
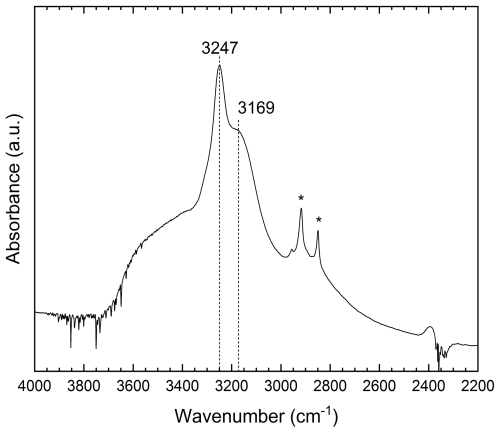
Figure 9Single-crystal FTIR spectra of C1 sample in the 2200–4000 cm−1 region. (*) Contamination during sample manipulation.
3.5 Infrared spectroscopy
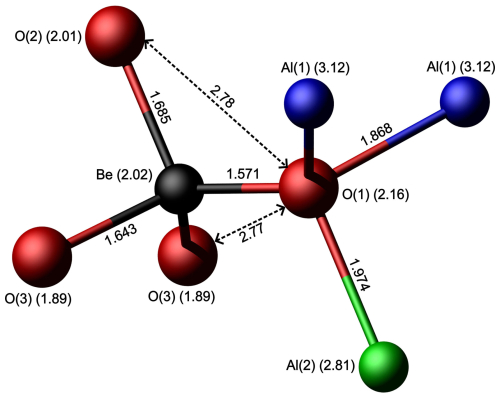
The infrared unpolarized absorption spectrum (Fig. 9) of the studied crystal shows two main absorptions: a sharp peak at 3247 cm−1 and a pronounced shoulder centred at around 3169 cm−1. These bands are superimposed on a broad absorption band spanning from 2600 to 3800 cm−1. These bands are attributable to the absorption of O–H stretching vibrations, as reported by Bauerhansl and Beran (1997) in chrysoberyl (3234–3219 and 3135 cm−1) and Malsy and Armbruster (2012) (3230 and 3130 cm−1) in alexandrite. In detail, the observed bands are shifted to higher wavenumber compared to those reported by these authors. The band at 2850 cm−1 described by Bauerhansl and Beran (1997) and Malsy and Armbruster (2012) is not observable in our spectrum as it is probably hidden beneath some contamination absorption. The broad absorption could be attributed to fluid inclusions within the sample or to disorder in the structural position of the OH defect. Bauerhansl and Beran (1997), based on polarized infrared spectra of chrysoberyl, determined that the OH dipole is oriented parallel to [001] ([100] in their crystallographic orientation). They explained the presence of OH groups by assuming vacancies in the cationic lattice, specifically at the Be site, with a possible influence of substituting cations at the Al(1) and Al(2) sites and their oxidation states (e.g. Fe). Their conclusion is that O(1) oxygens are partially replaced by OH groups, which point directly to vacancies at the Be sites, with O(1) acting as the main donor oxygen for the OH groups (Fig. 10). According to Libowitzky (1999), observed stretching frequencies are compatible with the presence of an enhanced H bonding. Applying the Libowitzky (1999) correlations, i.e.
for the observed 3169 and 3247 cm−1 frequencies results in O(1)–O(?) donor–acceptor distances of 2.69 and 2.73 Å, respectively, and the O–H…O distances are 1.83 and 1.88 Å, respectively. The donor–acceptor distances show a fairly good match with observed O(1)–O(3) and O(1)–O(2) distances of 2.77 and 2.78 Å, respectively (Fig. 10). Considering the bond valence distribution between O(2) and O(3) oxygens, it could be hypothesized that O(3) is the main acceptor anion. The higher wavenumber observed in Trevignano chrysoberyl compared with those reported by Bauerhansl and Beran (1997) for typical Fe-poor chrysoberyl can be explained by the influence of the larger Fe+3 in the Al(2) site which can shorten the O(1)–H bond, thus shifting the absorption bands to higher frequencies.
Based on the observed data, the Trevignano chrysoberyl exhibits unique characteristics not previously documented in the literature. In detail, this chrysoberyl is distinguished by three specifics features:
-
Chemical peculiarity and petrological implication.
-
Anomalous cell dimensions, bond lengths, and iron content.
-
Mineralogical association and genetic mode.
4.1 Chemical peculiarity and petrological implication
Literature data show that total Fe2O3 contents in chrysoberyl are mostly in the range 0.53 wt %–1.85 wt %, with some rare occurrence where Fe2O3≅2 wt %–4 wt % and, exceptionally, up to 6.25 wt % (Žáček and Vràna, 2002, and subsequent literature). The highest iron contents are generally reported for chrysoberyl samples from metamorphic rocks, with Fe2O3 typically in the 2.5 wt % to 3.2 wt % range (Downes and Bevan, 2002), while from metamorphosed pegmatites it is about 1.5 wt %–2.0 wt % (Franz and Morteani, 1984) and 0.67 wt %–1.3 wt % from granitic pegmatites (González del Tánago, 1991; Soman and Nair, 1985). This trend could suggest a progressive increase in Fe content with increase in metamorphic grade (Merino et al., 2013; Rybnikova et al., 2023). However, this correlation does not fit well with our chrysoberyl, whose Fe2O3 content (up to 17.24 wt %) would indicate, following Merino et al. (2013) and Rybnikova et al. (2023), a high metamorphic grade. Indeed, in the same work Merino et al. (2013) also reported that high Ti and low Cr contents are typical for igneous chrysoberyl. LA-ICP-MS and SEM-WDS data show a variable but generally exceptionally high Ti content (TiO2 up to 1.89 wt %) and Cr below detection limit, inferring at the same time a typical igneous origin.
Textural characteristics of these samples, particularly oscillatory zoning, suggest a third possible hybrid genetic mode. In fact, oscillatory zoning (shared by associated minerals, such as zircons) can be explained by metasomatism. The alternance of -rich zones can be ascribed to mineral growth under supersaturated conditions due to fluid infiltration (Yardley et al., 1991), fitting well with geological evidence of the Sabatini complex, well known for the presence of geothermal sulfatic brine circulation (De Rita et al., 1996; Cavarretta and Tecce, 1987).
This chemical and petrological information adds important insights for a more constrained characterization of the sanidinites which are still not well characterized yet, suggesting a high equilibrium temperature and pervasive fluid circulation related to infiltration metasomatism.
Table 11Chrysoberyl cell parameters for different Fetot contents.
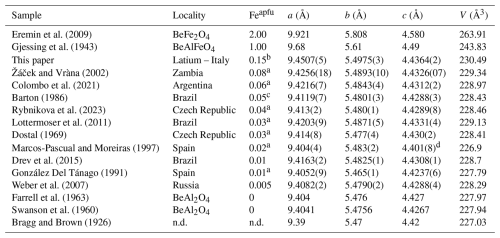
Eremin et al. (2009) calculated data; Gjessing et al. (1943), Swanson et al. (1960) and Farrell et al. (1963) synthetic (probably same data and/or the same material); a apfu from average calculated formula; b apfu from refined site occupancy; c recalculated; d questionable data.
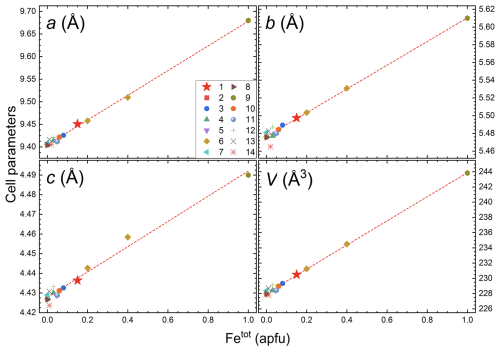
Figure 11Cell parameters versus Fetot (apfu) plot of selected literature data: (1) Trevignano; (2) Farrell et al. (1963); (3) Žáček and Vràna (2002); (4) Dostal (1969); (5) Rybnikova et al. (2023); (6) Tabata et al. (1981); (7) Bragg and Brown (1926); (8) Swanson et al. (1960); (9) Gjessing et al. (1943); (10) Colombo et al. (2021); (11) Barton (1986); (12) Lottermoser et al. (2011); (13) Drev et al. (2015); (14) González Del Tánago (1991). The red line represents the trend line of refined, synthetic, and experimental samples.
4.2 Anomalous cell dimensions, bond lengths, and iron content
Among the worldwide occurrences of chrysoberyl, the studied sample exhibits the largest unit-cell parameters observed in nature to date (Table 11), resulting from the chemical expansion induced by Fe-to-Al substitution. In fact, comparing the lattice parameters and Fetot content of the Trevignano chrysoberyl with literature data, including experimental results from Tabata et al. (1981) on Fe-doped synthetic chrysoberyl (Fig. 11), a clear positive linear correlation emerges between cell dimensions and Fetot content (Žáček and Vràna, 2002; Newnham et al., 1964). This linearity is also confirmed by considering the unit-cell parameters calculated for the ideal BeFe2O4 phase by Eremin et al. (2009, Table 11).
The exceptionally high iron content in Trevignano chrysoberyl must be the result of both an iron-rich oxidizing formation environment and from physical factors such as unusually elevated temperatures. Indeed, higher temperature could facilitate Fe-to-Al substitution, as Fe3+ (ionic radius 0.649 Å) is larger than Al3+ (ionic radius 0.537 Å) (Hawthorne and Gagné, 2024), and the expansion associated with the rise in temperature may enhance the incorporation of iron in the structure. The rarity of Fe2O3 contents exceeding 2 wt % in other worldwide occurrences suggests that although the abundance of Fe3+ is a necessary condition for enrichment it is not sufficient. This indicates that both geochemical and physical factors are crucial for such enrichment.
Table 12Comparison between refined bond lengths and Hazen and Finger (1987) for chrysoberyl thermal expansion.

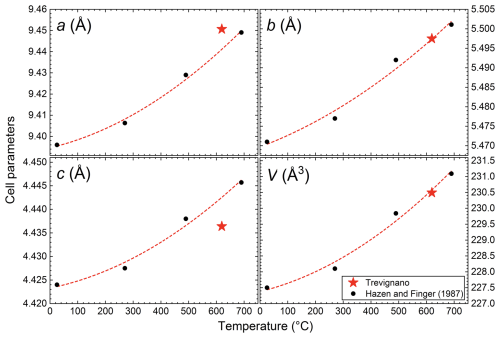
Figure 12Cell parameters versus temperature plot: black points represent Hazen and Finger (1987) refined cell parameters with increasing temperature at 25, 270, 490, and 690 °C. The trend line shows Hazen and Finger (1987) observed polynomial correlation (only black point fitted). The red star represents the refined cell parameter for the Trevignano sample, corresponding to an estimated temperature of 620 °C.
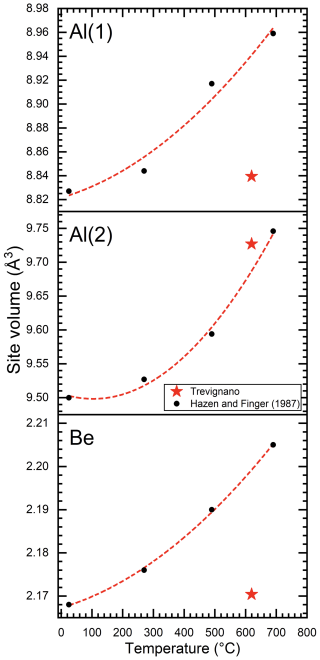
Figure 13Al(1), Al(2), and Be polyhedral site volumes versus temperature. Black points represent the Hazen and Finger (1987) refined site volume with increasing temperature at 25, 270, 490, and 690 °C. The red line represents the Hazen and Finger (1987) correlation (only black point fitted). The red star represents site volumes for Trevignano sample, corresponding to an estimated temperature of 620 °C. The polyhedral volumes were determined by using Vesta software (Momma and Izumi, 2011).
If we consider the temperature as the primary factor controlling the structural expansion necessary for this iron accommodation, using the correlation between unit-cell volume and temperature derived from Hazen and Finger (1987, Table 12), an analogous volume of Trevignano chrysoberyl is raised at 620 °C (V=230.49 Å3; Fig. 12). In effect, this temperature aligns well with data from the degree of ordering in sanidine associated with chrysoberyl (work in preparation), indicating a medium-to-high sanidine equilibrium temperature exceeding 550 °C (Della Ventura et al., 1992; Brown and Parsons, 1989).
A comparison between chrysoberyl thermal expansion and the refined structure (Figs. 12, 13) emphasizes how the differential chemical expansion resulting from Fe-to-Al substitution at the sole Al(2) site differs from the isotropic expansion induced by increasing temperature, as well as how the different behaviours of the Al(1), Al(2), and Be sites may provide valuable insights into the distribution of Fe within the Al(2) site, as confirmed by structural refinement. Notably, while Al(1) and Be polyhedral site volumes (Fig. 13) are, as expected, completely out of trend since they are not influenced by Fe, the cell parameters of the Trevignano chrysoberyl deviate only slightly compared with the experimental data for the analogous volume derived from thermal expansion reported by Hazen and Finger (1987) (Fig. 12). These observations suggest that metal incorporation induces a certain degree of structural deformation, which warrants further investigation to understand its effects on the mechanical and optical properties of doped chrysoberyl.
The clues about Fe partitioning are particularly significant, given this has been a subject of debate in the past. The studies of Newnham et al. (1964), based on the structural refinement of Farrell et al. (1963), enabled Newnham et al. (1964) to evaluate up to 50 % Fe-to-Al substitution, demonstrating that Fe3+ preferentially populates the larger Al(2) site. This finding contradicted earlier assumptions by Vinokurov et al. (1962), which suggested that Fe3+ exclusively occupies the inversion symmetry sites (i.e. Al(1)). A subsequent research by Gromalova et al. (2011) further confirmed the preferential occupancy of Fe3+ at the Al(2) site through stability region calculation for the solid solution and determination of critical temperature and composition across the entire compositional range. Lottermoser et al. (2011), although studying a low iron content chrysoberyl (Fe = 0.03 apfu), examined local expansion of coordination octahedra and concluded that Fe3+ ions could theoretically replace Al on both Al(1) and Al(2) sites. However, they acknowledged that attempts to refine a model with Fe3+ distributed across both sites were unsuccessful.
Our structural refinement, supported by the comparison between chemical and thermal expansion data for natural Fe-bearing chrysoberyl, corroborates the conclusion of Newnham et al. (1964) and Gromalova et al. (2011), confirming that Fe3+ is preferentially partitioned into the larger Al(2) site.
Regarding cell parameters, both considerations (thermal and crystal-chemical, Figs. 12, 11) converge in a slight deviation regard a and c refined lengths, bigger and smaller, respectively. These deviations from the linear trend are probably due to the different distribution of Al(1) and Al(2) sites along the a and c axes. In particular, Al(1) polyhedra are distributed sharing edges along b axes and being very little affected directly by iron content they constitute a constraint on cell expansion and are in line, as volume, with an expected linear expansion.
4.3 Mineralogical association and genetic mode
Chrysoberyl deposits and occurrences were extensively studied by Beus (1966) and Hawkesworth (2003). Franz and Morteani (1984, 2002) proposed two primary mechanisms for chrysoberyl formation. The first, and most widely accepted, involves the breakdown of primary beryl into chrysoberyl and quartz, accounting for the characteristic paragenesis observed in chrysoberyl occurrences worldwide. The second mechanism considers the formation of chrysoberyl and phenakite as products of a reaction between alkali feldspar and beryl under hydrothermal conditions. However, the absence of beryl, quartz and phenakite in our samples raises doubts about these proposed secondary origins, supporting the idea of direct crystal growth, likely from Be-rich fluids.
Furthermore, specific geologic conditions must be met for chrysoberyl formation and persistence. According to Černý (2002), the relative rarity of chrysoberyl is due to its reaction with K-feldspar at decreasing temperature and increasing H2O, leading to the formation of beryl and muscovite, a typical pegmatitic association.
Given the composition of these ejecta, which consists almost entirely of K-feldspar, the absence of quartz likely played a key role in the presence of chrysoberyl and the subsequent absence of beryl and Al-mica crystals. In fact, Beus (1966), Franz and Morteani (1984, 2002) and Černý (2002), emphasize the importance of potential “desilication” (or the equivalent alumina enrichment) in the rocks for chrysoberyl stability. In the geological context of the RR, this process likely occurred through metasomatic exchange with the thick pre-volcanic carbonate basement of the Sabatini district. Indeed, such metasomatic ejecta (skarn) are commonly found within the pyroclastic deposits of the region. It is proposed that the formation of chrysoberyl in this setting was facilitated by the pervasive infiltration and circulation of extremely hot, silica-undersaturated fluids within the carbonate basement. This thermal and chemical perturbation of the host rock likely provided the necessary conditions for the nucleation and growth of chrysoberyl crystals.
The exceptional iron-rich chrysoberyl occurrences documented in volcanic ejecta of the Sabatini Volcanic Complex (central Italy) provide unique insights into their structural features, such as the complete partitioning of Fe3+ into the larger Al(2) site. Structural analyses emphasize the significant role of metal substitution in both chemical expansion and differential polyhedral deformation, as well as its impact on the optical properties of chrysoberyl, which are particularly relevant for its industrial, medical, and military laser applications.
The absence of typical chrysoberyl-associated minerals, such as quartz, phenakite, beryl, and other aluminium silicates (e.g. Al2SiO5), suggests a distinct formation mechanism, likely involving direct crystallization from a fluid-rich environment. This hybrid igneous-metasomatic origin, driven by high temperatures and pervasive fluid circulation, is supported by textural, optical, and chemical evidence. Furthermore, this process appears capable of accommodating significantly higher amounts of metals, warranting further investigation to explain this anomaly.
Lastly, considering the well-documented respiratory toxicity of beryllium (Pawlas and Pałczyński, 2022), this finding highlights an important geogenic source of this element in the subsurface of the Sabatini Volcanic Complex and underscores the need for a comprehensive assessment of beryllium distribution in the soils of the Bracciano area. Such an evaluation, building on previous work in the Vico volcanic complex (Armiento et al., 2013), is crucial to understanding the potential health risks associated with beryllium exposure in this region.
Data are available on request from the authors.
The supplement related to this article is available online at https://doi.org/10.5194/ejm-37-483-2025-supplement.
GI: discovery of chrysoberyl samples, conceptualization, investigation, and manuscript draft writing; FB: supervision, investigation, and data and figure curation; FB and CB: content and logical validation of the manuscript draft; SM and CB: single-crystal XRD measurements and structural refinement; FB and GI: analyses of IR and chemical data; AR: acquisition and analysis of LA-ICP-MS data; EC and GI: optical analyses; MEC: preliminary identification by SEM-EDS and micro-Raman; FB, CB, AR, EC, and MEC: final validation and revision of the manuscript draft.
At least one of the (co-)authors is a member of the editorial board of European Journal of Mineralogy. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Celebrating the outstanding contribution of Paola Bonazzi to mineralogy”. It is not associated with a conference.
The authors would like to express their gratitude to Luca Tortora and Valerio Graziani from the LASR3 laboratory for performing the infrared spectroscopy analyses. Special thanks to Andrea Cavallo and Luca Del Rio from the CERTEMA laboratory in Grosseto for conducting the microanalyses, to Erica Bittarello for her assistance in preliminary identification of chrysoberyl in a Trevignano specimen lot, and to Ezio Curti for providing one of the chrysoberyl samples analysed in this study (C6).
This paper was edited by Dmitry Pushcharovsky and reviewed by Peter Bacik and one anonymous referee.
Anthony, J. W., Bideaux, R. A., Bladh, K. W., and Nichols, M. C.: Handbook of Mineralogy, Min. Soc. Am., 5, https://www.handbookofmineralogy.org (last access: March 2025), 2003.
Armiento, G., Bellatreccia, F., Cremisini, C., Della Ventura, G., Nardi, E., and Pacifico, R.: Beryllium natural background concentration and mobility: a reappraisal examining the case of high Be-bearing pyroclastic rocks, Environ. Monit. Assess., 185, 559–572, https://doi.org/10.1007/s10661-012-2575-3, 2013.
Barton, M. D.: Phase equilibria and thermodynamic properties of minerals in the BeO-Al2O3-SiO2-H2O (BASH) system, with petrologic application, Am. Mineral., 71, 277–300, 1986.
Bauerhansl, P. and Beran, A.: Trace hydrogen in the olivine-type minerals chrysoberyl, Al2BeO4 and sinhalite, MgAlBO4 – a polarized FTIR spectroscopic study, Schweiz. Miner. Petrog., 77, 131–136, 1997.
Beus, A. A.: Geochemistry of Beryllium and genetic types of Beryllium deposits, edited by: Freeman, W. H., San Francisco, 401, 1966.
Bouzari, N., Tabatabai, H., Abbasi, Z., Firooz, A., and Dowlati, Y.: Laser hair removal: comparison of long-pulsed Nd:YAG, long-pulsed alexandrite, and long-pulsed diode lasers, Dermatol. Surg., 30, 498–502, https://doi.org/10.1111/j.1524-4725.2004.30163.x, 2004.
Bragg, W. L. and Brown, G. B.: The crystalline structure of chrysoberyl, Proc. R. Soc. Lond. A., 110, 34–63, https://doi.org/10.1098/rspa.1926.0003, 1926.
Brown, W. L. and Parsons, I.: Alkali feldspars: ordering rates, phase transformations and behaviour diagrams for igneous rocks, Mineral. Mag., 53, 25–42, https://doi.org/10.1180/minmag.1989.053.369.03, 1989.
Bruker AXS Inc.: APEX4. Bruker Advanced X-ray Solutions, Madison, Wisconsin, USA, 2022.
Bukin, G. V., Matrosov, V. N., Orekhova, V. P., Remigailo, Yu. L., Sevastyanov, B. K., Syomin, E. G., Solntsev, V. P., and Tsvetkov, E. G.: Growth of alexandrite crystals and investigation of their properties, J. Cryst. Growth, 52, 537–541, https://doi.org/10.1016/0022-0248(81)90335-3, 1981.
Cámara, F., Oberti, R., Ottolini, L., Della Ventura, and Bellatreccia, G. F.: The crystal chemistry of Li in gadolinite, Am. Mineral., 93, 996–1004, https://doi.org/10.2138/am.2008.2748, 2008.
Cavarretta, G. and Tecce, F.: Contact metasomatic and hydrothermal minerals in the SH2 deep well, Sabatini volcanic district, Latium, Italy, Geothermics, 16, 127–145, https://doi.org/10.1016/0375-6505(87)90061-7, 1987.
Černý, P.: Mineralogy of beryllium in granitic pegmatites, in: Beryllium: Mineralogy, Petrology and Geochemistry, edited by: Grew, E. S., Rev. Mineral. Geochem., 50, 405–444, https://doi.org/10.1515/9781501508844-011, 2002.
Černý, P., Novák, M., and Chapman, R.: Effects of sillimanite-grade metamorphism and shearing on Nb-Ta oxide minerals in granitic pegmatites: Maršíkov, Northern Moravia, Czechoslovakia, Can. Mineral., 30, 699–718, 1992.
Conticelli, S. and Peccerillo, A.: Petrology and geochemistry of potassic and ultrapotassic volcanism in central Italy: petrogenesis and inferences on the evolution of the mantle sources, Lithos, 28, 221–240, https://doi.org/10.1016/0024-4937(92)90008-M, 1992.
Colombo, F., Sfragulla, J., González Del Tánago, J., and Pannunzio Miner, E. V.: Crisoberilo de la pegmatita Tablata I, Grupo pegmatitico Pocho, distrito Altautina (provincia de Córdoba, Argentina), Revista de la Asociación Geológica Argentina, 78, 344–354, 2021.
Cygan, G. L., Chou, I. M., and Sherman, D. M.: Reinvestigation of the annite = sanidine + magnetite + H2 reaction using the fH2 sensor technique, Am. Mineral., 81, 475–485, 1996.
Della Ventura, G., Di Lisa, G. A., Marcelli, M., Mottana, A., and Paris, R.: Composition and structural state of alkali feldspars from ejecta in the Roman potassic province, Italy; petrological implications, Eur. J. Mineral., 4, 411–424, https://doi.org/10.1127/ejm/4/3/0411, 1992.
Della Ventura, G., Rossi, P., Parodi, G. C., Mottana, A., Rausepp, M., and Prencipe, M.: Stoppaniite, (Fe,Al,Mg)4(Be6Si12O36)*(H2O)2(Na,□) a new mineral of the beryl group from Latium (Italy), Eur. J. Mineral., 12, 121–127, https://doi.org/10.1127/0935-1221/2000/0012-0121, 2000.
Della Ventura, G., Bonazzi, P., Oberti, R., and Ottolini, L.: Ciprianiite and mottanaite-(Ce), two new minerals of the hellandite group from Latium (Italy), Am. Mineral., 87, 739–744, https://doi.org/10.2138/am-2002-5-617, 2002.
De Rita, D., Funiciello, R., Rossi, U., and Sposato, A.: Structure and evolution of the Sacrofano-Baccano caldera, Sabatini volcanic complex, Rome, J. Volcanol. Geoth. Res., 17, 219–236, https://doi.org/10.1016/0377-0273(83)90069-0, 1983.
De Rita, D., Funiciello, R., Corda, L., Sposato, A., and Rossi, U.: Volcanic Units, in: Sabatini Vulcanic Complex, edited by: Di Filippo, M., Progetto finalizzato di Geodinamica, Quaderni de La Ricerca Scientifica, Monografie finali, 11, 33–79, 1993.
De Rita, D., Di Filippo, M., and Rosa, C.: Structural evolution of the Bracciano volcano-tectonic depression, Sabatini volcanic complex, Italy, Geological Society special publication, 110, 225–236, https://doi.org/10.1144/gsl.sp.1996.110.01.17, 1996.
De Rita, D., Rodani, S., Rosa, C., and Puzzilli, L.: Il settore sud-occidentale del distretto vulcanico sabatino: stratigrafia ed evoluzione alla luce di dati di sondaggio e di rilevamento, Bollettino della Società Geologica Italiana, 116, 319–334, 1997.
Di Filippo, M. (Ed.): Sabatini volcanic complex, Quaderni de La Ricerca Scientifica, Monografie finali, 11, 9–109, 1993.
Dostal, J.: Some new data for chrysoberyl from Maršíkov, Acta U. Carol. Geol., 4, 261–270, 1969.
Downes, P. J. and Bevan, A. W. R.: Chrysoberyl, beryl and zincian spinel mineralization in granulite facies at Dowerin, Western Australia, Mineral. Mag., 66, 985–1002, https://doi.org/10.1180/0026461026660072, 2002.
Drev, S., Komelj, M., Mazaj, M., Daneu, N., and Rečnik, A.: Structural investigation of (130) twins and rutile precipitates in chrysoberyl crystals from Rio das Pratinhas in Bahia (Brazil), Am. Mineral., 100, 861–871, https://doi.org/10.2138/am-2015-5120, 2015.
Droop, G. T. R.: A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria, Mineral. Mag., 51, 431–435, https://doi.org/10.1180/minmag.1987.051.361.10, 1987.
Eremin, N. N., Gromalova, N. A., and Urusov, V. S.: Atomic modeling and prediction of the structure, energy characteristics of point defects, and thermodynamic and elastic properties of the simple and complex beryllium oxides, Glass Phys. Chem., 35, 613–619, https://doi.org/10.1134/S1087659609060108, 2009.
European Commission: Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, in: Study on the critical raw materials for the EU 2023: final report, edited by: Grohol, M. and Veeh, C., Publications Office of the European Union, https://doi.org/10.2873/725585, 2023.
Farrell, E. F., Fang, J. H., and Newnham, R. E.: Refinement of the chrysoberyl structure, Am. Mineral., 48, 804–810, 1963.
Fibrich, M., Šulc, J., Vyhlídal, D., Jelínková, H., and Čech, M.: Alexandrite spectroscopic and laser characteristic investigation within 78–400 K temperature range, Laser Phys., 27, 115801, https://doi.org/10.1088/1555-6611/aa884c, 2017.
Franz, G. and Morteani, G.: The formation of chrysoberyl in metamorphosed pegmatites, J. Petrol., 25, 27–52, https://doi.org/10.1093/petrology/25.1.27, 1984.
Franz, G. and Morteani, G.: Be-Minerals: Synthesis, Stability and Occurrence in Metamorphic Rocks, in: Beryllium: Mineralogy, Petrology and Geochemistry, edited by: Grew, E. S., Rev. Mineral. Geochem., 50, 552–589, https://doi.org/10.1515/9781501508844-014, 2002.
Funiciello, R., Parotto, M., De Rita, D., Di Filippo, M., and Sposato, A.: Carta geologica del Complesso vulcanico sabatino. con note illustrative. Consiglio Nazionale delle Ricerche, Progetto finalizzato Geodinamica, Sottoprogetto 3: Sorveglianza dei vulcani attivi e rischio vulcanico, Comitato redazionale C.N.R., Ed., Roma, 1988.
Gagné, O. C. and Hawthorne, F. C.: Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen, Acta Crystallogr., B71, 562–578, 2015.
Gao, Y., Li, X., Cheng, Y., Huang, T., Li, K., Xu, B., and Tang, R.: Gemological, Spectral and Chemical Features of Canary Yellow Chrysoberyl, Crystals, 13, 1580, https://doi.org/10.3390/cryst13111580, 2023.
Gjessing, L., Larsson, T., and Major, H.: lsomorphous Substitute for Al3+ in the compound Al2BeO4, Norsk Geol Tidssk, 22, 92–99, 1943.
González del Tánago, J.: Las pegmatitas graníticas de Sierra Albarrana (Córdoba, España): Mineralizaciones de berilio, Boletín Geológico y Minero de España 102, 90–114, https://hdl.handle.net/20.500.14352/60256, 1991.
Gromalova, N. A., Eremin, N. N., and Urusov, V. S.: Atomistic modeling of the mixing properties and local structure of Be(Al,Cr,FeIII)2O4 solid solutions, Glass Phys. Chem., 3, 293–306, https://doi.org/10.1134/S1087659611030059, 2011.
Gromalova, N. A., Mal'tsev, V. V., Dorokhova, G. I., Leonyuk, N. I., and Urusov, V. S.: Recrystallization of natural chrysoberyl in multicomponent melts, Crystallog. Rep., 57, 603–608, https://doi.org/10.1134/S1063774512030078, 2012.
Hawkesworth, C. J.: Beryllium: Mineralogy, Petrology, and Geochemistry, edited by: Grew, E. S., Rev. Mineral. Geochem., 50, Washington, D.C, USA, https://doi.org/10.1180/0670824, 2003.
Hawthorne, F. C. and Gagné, O. C.: New ion radii for oxides and oxysalts, fluorides, chlorides and nitrides, Acta Cryst., B80, 326–339, https://doi.org/10.1107/S2052520624005080, 2024.
Hazen, R. M. and Finger, L. W.: High-temperature crystal chemistry of phenakite (Be2SiO4) and chrysoberyl (BeAl2O4), Phys. Chem. Miner., 14, 426–434, https://doi.org/10.1007/BF00628819, 1987.
Jochum, K. P., Weis, U., Stoll, B., Kuzmin, D., Yang, Q., Raczek, I., Jacob, D. E., Stracke, A., Birbaum, K., and Frick, D. A.: Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines, Geostand. Geoanal. Res., 35, 397–429, https://doi.org/10.1111/j.1751-908X.2011.00120.x, 2011.
Kanouo, N. S., Ekomane, E., Yongue, R. F., Njonfang, E., Zaw, K., Ma, C., Ghogomu, T. R., Lentz, D., and Venkatesh, A. S.: Trace elements in corundum, chrysoberyl, and zircon: Application to mineral exploration and provenance study of the western Mamfe gem clastic deposits (SW Cameroon, Central Africa), J. Afr. Earth Sci., 113, 35–50, https://doi.org/10.1016/j.jafrearsci.2015.09.023, 2016.
Landthaler, M. and Hohenleutner, U.: Laser therapy of vascular lesions, Photodermatol. Photoimmunol. Photomed., 22, 324–332, https://doi.org/10.1111/j.1600-0781.2006.00254.x, 2006.
Li, L., Kono, T., Groff, W. F., Chan, H. M., Kitazawa, Y., and Nozaki, N. J.: Comparison study of a long-pulse pulsed dye laser and a long-pulse pulsed alexandrite laser in the treatment of port wine stains, J. Cosmet. Laser. Ther., 10, 12–15, https://doi.org/10.1080/14764170701817023, 2008.
Libowitzky, E.: Correlation of O-H stretching frequencies and O-H…O hydrogen bond lengths in minerals, Monatsh. Chem., 130, 1047–1059, https://doi.org/10.1007/BF03354882, 1999.
London, D. and Evensen, J. M.: Beryllium in Silicic Magmas and the Origin of Beryl-Bearing Pegmatites, in: Beryllium: Mineralogy, Petrology and Geochemistry, edited by: Grew, E. S., Rev. Mineral. Geochem., 50, 445–486, https://doi.org/10.1515/9781501508844-012, 2002.
Lottermoser, W., Redhammer, G. J., Weber S. U., Litterst, F. J., Tippelt, G., Dlugosz, S., Bank, H., Amthauer, G., and Grodzicki, M.: The electric field gradient in natural iron-doped chrysoberyl Al2BeO4 and sinhalite MgAlBO4 single crystals, Phys. Chem. Miner., 38, 787–799, https://doi.org/10.1007/s00269-011-0451-2, 2011.
Malsy, A. K. and Armbruster, T.: Synthetic alexandrite – Growth methods and their analytical fingerprints, Eur. J. Mineral., 24, 153–162, https://doi.org/10.1127/0935-1221/2012/0024-2181, 2012.
Mandarino, J. A.: The Gladstone-Dale relationship. Part I: derivation of new constants, Can. Mineral., 14, 498–502, 1976.
Mandarino, J. A.: The Gladstone-Dale relationship: Part III. Some general applications, Can. Mineral., 17, 71–46, 1979.
Marcos-Pascual, C. and Moreiras, D. B.: Characterization of alexandrite, emerald and phenakite from Franqueira (NW Spain), J. Gem., 25, 340–357, https://doi.org/10.15506/JoG.1997.25.5.340, 1997.
Merino, E., Villaseca, C., Perez-Soba, C., and Orejana, D.: First occurrence of Gahnite and chrysoberyl in an Iberian Hercynian pluton: the Belvis de Monroy granite (Careres, Spain), Macla, Revista de la Sociedad espanola de mineralogia, 13, 159–160, 2010.
Merino, E., Villaseca, C., Orejana, D., and Jeffries, T.: Gahnite, hrysoberyl and beryl co-occurrence as accessory minerals in highly evolved peraluminous pluton: The belvìs de Monroy leucogranite (Càceres, Spain), Lithos, 179, 137–156, https://doi.org/10.1016/j.lithos.2013.08.004, 2013.
Momma, K. and Izumi, F.: VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Cryst., 44, 1272–1276, https://doi.org/10.1107/S0021889811038970, 2011.
Newnham, R. E., Santoro, R., Pearson, J., and Jansen, C.: Ordering of Fe and Cr in chrysoberyl, Am. Mineral., 49, 427–430, 1964.
Oberti, R., Langone, A., Boiocchi, M., Bernabè, E., and Hawthorne, F. C.: News from the hellandite group: the redefinition of mottanaite and ciprianiite and the new mineral description of ferri-mottanaite-(Ce), the first Fe3+-dominant hellandite, Eur. J. Mineral., 31, 799–806, https://doi.org/10.1127/ejm/2019/0031-2858, 2019.
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J.: Iolite: Freeware for the visualisation and processing of mass spectrometric data, J. Anal. Atom. Spectrom., 26, 2508–2518, https://doi.org/10.1039/C1JA10172B, 2011.
Pawlas, N. and Pałczyński, C. M.: Beryllium, in: Handbook on the Toxicology of Metals (Fifth Edition), Vol. II: Specific Metals, 101–119, https://doi.org/10.1016/B978-0-12-822946-0.00004-0, 2022.
Peccerillo, A.: Plio-Quaternary Volcanism in Italy: Petrology, Geochemistry, Geodynamics, Springer Berlin, Heidelberg, https://doi.org/10.1007/3-540-29092-3, 2005.
Peccerillo, A.: Cenozoic Volcanism in the Tyrrhenian Sea Region, Advances in Volcanology, Springer Cham., 399, https://doi.org/10.1007/978-3-319-42491-0, 2016.
Rabiee, A., Rossetti, F., Lustrino, M., Azizi, H., Asahara, Y., Alipour, S., and Selby, D.: Formation and degradation of a porphyry occurrence: The Oligocene Khatoon-Abad porphyry Mo-Cu system, NW Iran, Ore Geol. Rev., 174, 106330, https://doi.org/10.1016/j.oregeorev.2024.106330, 2024.
Rossi, P., Bellatreccia, F., Caprilli, E., Parodi, G., Della Ventura, G., and Mottana, A.: A new occurrence of rare minerals in an ejectum in the pyroclastics of Vico Volcano, Roman Comagmatic Region, Italy, Rend. Fis. Acc. Lincei, 6, 147–156, https://doi.org/10.1007/BF03001663, 1995.
Rybnikova, O., Uher, P., Novák, M., Chládek, Š., Bacik, P., Kurylo, S., and Vaculovic, T.: Chrysoberyl and associated beryllium minerals resulting from metamorphic overprint of the Maršíkov-Schinderhübel-III pegmatite, Czech Republic, Mineral. Mag., 87, 369–381, https://doi.org/10.1180/mgm.2023.22, 2023.
Schmetzer, K., Bernhardt, H. J., and Hainschwang, T.: Flux-grown synthetic alexandrites from Creative Crystals Inc., J. Gem., 33, 49–81, https://doi.org/10.15506/JoG.2012.33.1.49, 2012.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Cryst., C71, 3–8, https://doi.org/10.1107/s2053229614024218, 2015.
Soman, K. and Nair, N. G. K.: Genesis of chrysoberyl in the pegmatites of Southern Kerala, India, Mineral. Mag., 733–738, https://doi.org/10.1180/minmag.1985.049.354.14, 1985.
Sottili, G., Palladino, D. M., Marra, F., Jicha, B., Karner, D. B., and Renne, P.: Geochronology of the most recent activity in the Sabatini Volcanic District, Roman Province, central Italy, J. Volcanol. Geoth. Res., 196, 20–30, https://doi.org/10.1016/j.jvolgeores.2010.07.003, 2010.
Steven, C. J. and Gunter, M. E.: Excelibr: an excel spreadsheet for solving the optical orientation of uniaxial and biaxial crystals, The Microscope, 65, 147–152, 2017.
Šulc, J. and Jelínková, H.: Solid-state lasers for medical application, in: Laser for Medical application, 1st edn., edited by: Jelínková, H., Woodhead Publishing Series in Electronic and Optical Materials, Elsevier BV, 127–176, https://doi.org/10.1533/9780857097545.2.127, 2013.
Sun, Z., Palke, A. C., Muyal, J., DeGhionno, D., and McClure, S. F.: Geographic origin determination of alexandrite, Gems Gemol., 55, 660–681, https://doi.org/10.5741/GEMS.55.4.660, 2019.
Swanson, H. E., Cook, M. I., Issacs, T., and Evans, E. H.: Standard X-ray diffraction powder patterns, Bur. Std. Circ., 539, 9, 64 pp., 1960.
Tabata, H., Ishii, E., and Okuda, H.: Solid Solubilities in the System BeAl2O4-BeR2O4 (R=Cr, Fe, Ga), J. Ceram. Assoc., 89, 31–38, https://doi.org/10.2109/jcersj1950.89.31, 1981.
Tarquini S., Isola, I., Favalli, M., Battistini, A., and Dotta, G.: TINITALY, a digital elevation model of Italy with a 10 meters cell size (Version 1.1), INGV, https://doi.org/10.13127/tinitaly/1.1, 2023.
Vignola, P., Zucali, M., Rotiroti, N., Marotta, G., Risplendente, A., and Pavese, A.: The chrysoberyl- and phosphate-bearing albite pegmatite of Malga Garbella, Val Di Rabbi, Trento province, Italy, Can. Mineral., 56, 411–424, https://doi.org/10.3749/canmin.1700058, 2018.
Vinokurov, V. M., Zaripov, M. M., Stepanov, V. G., Pol'skii, Y. E., Cherkin, G. K., and Shekun, L. Y.: Electron paramagnetic resonance in natural chrysoberyl, Sov. Phys.-Sol. State (English translation), 3, 1797–1800, 1962.
Warr, L. N.: IMA–CNMNC approved mineral symbols, Mineral. Mag., 85, 291–320, https://doi.org/10.1180/mgm.2021.43, 2021.
Washington, H. S.: The Roman Comagmatic Region, Carnegie Institution for Science, Washington, D.C., USA, 57, p. 199, 1906.
Weber, S. U., Grodzicki, M., Lottermoser, W., Redhammer, G. J., Tippelt, G., Ponahlo, J., and Amthauer, G.: 57Fe Mössbauer spectroscopy, X-ray single-crystal diffractometry, and electronic structure calculations on natural alexandrite, Phys. Chem. Miner., 34, 507–515, https://doi.org/10.1007/s00269-007-0166-6, 2007.
Welberry, T. R. (Ed.): International Tables for Crystallography,Volume C: Mathematical, physical and chemical tables, second on-line edition, IUCr Series, International Tables for Crystallography, https://doi.org/10.1107/97809553602060000117, 2021.
Yardley, B. W., Rochelle, C. A., Barnicoat, A. C., and Lloyd, G. E.: Oscillatory zoning in metamorphic minerals: an indicator of infiltration metasomatism, Mineral. Mag., 55, 357–365, https://doi.org/10.1180/minmag.1991.055.380.06, 1991.
YinCe, M., Tao, H., ShanKe, L., Hang, L., Huadong, M., Wei, W., and Xingwang, X.: Types and genetic mechanism of chrysoberyl deposits, Acta Petrol. Sin., 38, 943–962, https://doi.org/10.18654/1000-0569/2022.04.01, 2022.
Žáček, V. and Vràna, S.: Iron-rich chrysoberyl from Kalanga Hill, Muyombe District, north-eastern Zambia, Neues Jb. Miner. Monat., 12, 529–540, https://doi.org/10.1127/0028-3649/2002/2002-0529, 2002.
HFSE: high‐field-strength element; LILE: large‐ion lithophile element; REE: rare earth element.