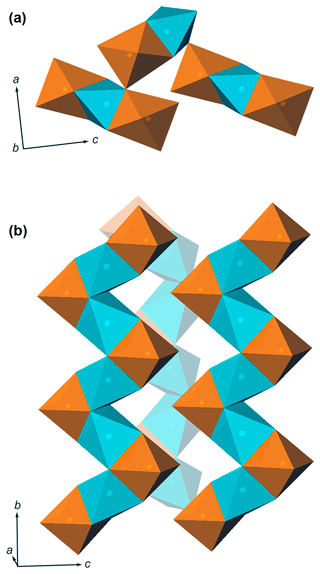the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
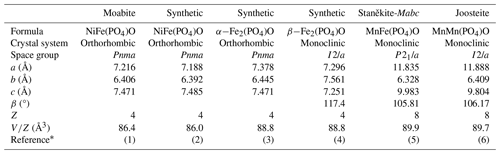
Moabite, NiFe3+(PO4)O, a new natural oxyphosphate
Mikhail N. Murashko
Maria G. Krzhizhanovskaya
Yevgeny Vapnik
Natalia S. Vlasenko
Oleg S. Vereshchagin
Dmitrii V. Pankin
Evgeny A. Vasiliev
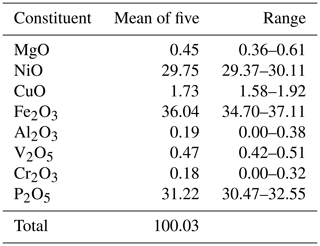
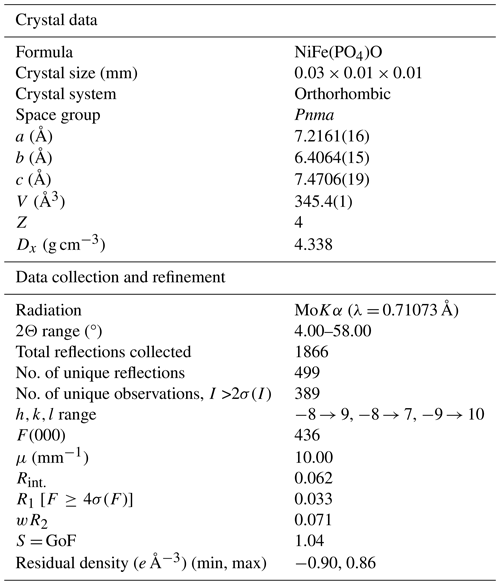
Moabite, NiFe3+(PO4)O, is a new natural oxyphosphate discovered in pyrometamorphic rocks of the Daba-Siwaqa complex, a subdivision of the Hatrurim Formation in central Jordan. The mineral is named for the Kingdom of Moab, an ancient state that existed on the territory of the modern Jordan. Moabite is an accessory phase in the phosphide–phosphate assemblages, where it associates with diopside; anorthite; crocobelonite, CaFe(PO4)2O; yakubovichite, CaNi2Fe3+(PO4)3; hematite; negevite, NiP2; murashkoite, FeP; transjordanite, Ni2P; halamishite, Ni5P4; native iron (α-Fe); and an alluaudite-group phosphate whose composition is exactly midway between the two endmembers NaNaCa(Fe3+Mg)(PO4)3 and □NaCa(Fe3+Fe3+)(PO4)3. The mineral forms isometric to short prismatic crystals and euhedral grains up to 30 µm across. Macroscopically, it has a deep-brown colour. In the polished sections in transmitted light, the mineral is translucent red-brown. It has a Mohs hardness rating of 4. Cleavage was not observed. The density, 4.324 g cm−3, was calculated based on the empirical formula and unit-cell parameters obtained from single-crystal refinement. The chemical composition was as follows (electron microprobe, wt %): NiO 29.75, CuO 1.73, MgO 0.45, Fe2O3 36.04, Al2O3 0.19, Cr2O3 0.18, V2O5 0.47, P2O5 31.22, total 100.03. The empirical formula calculated on the basis of 5 oxygen atoms per formula unit (apfu) is (Ni0.90Cu0.05Mg0.03)Σ0.98(Fe3+ 1.01Al0.01Cr0.01)Σ1.03(P0.99V5+ 0.01)Σ1.00O5, corresponding to the ideal NiFe3+(PO4)O. Moabite is orthorhombic; the space group is Pnma (no. 62); and a=7.2161(16), b=6.4064(15), c=7.4706(19) Å, V=345.4(1) Å3 and Z=4. The strongest lines of X-ray powder diffraction pattern are as follows [d in Å (I) (hkl)]: 5.20(63)(101), 3,321(37)(102), 3.251(83)(201), 2.7262(100)(121), 2.5946(37)(202), 2.3542(25)(103) and 2.3044(24)(122). The crystal structure has been solved and refined to R1=0.033 for 389 unique observed reflections. Moabite is the first mineral that crystallizes in the α-Fe2PO5 (α-Fe2OPO4) structure type. It has a direct synthetic analogue, and it is isotypic to antiferromagnetic transition metal oxyphosphates of the general formula OPO4, where Fe, Ni, Co and Cu and Fe, V and In.
- Article
(1841 KB) - Full-text XML
-
Supplement
(267 KB) - BibTeX
- EndNote
The Hatrurim Formation, a large complex of pyrometamorphic rocks in the Middle East (Gross, 1977), is distinguished by the unprecedented diversity of phosphorus-bearing minerals. They include a palette of silicophosphates and carbonate phosphates (Galuskin et al., 2015, 2016, 2018a, b, 2019, 2021; Sokol et al., 2015), anhydrous orthophosphates (Britvin et al., 2021c, 2023b; Galuskin et al., 2023a, 2024; Juroszek et al., 2023; Krzątała et al., 2023), phosphides (Britvin et al., 2015, 2020a, b, c, 2021b, 2022a; Murashko et al., 2022; Galuskin et al., 2023b), condensed phosphates (Britvin et al., 2021d), and oxyphosphates (Galuskin et al., 2018b, 2021; Britvin et al., 2023a). The majority of these minerals are endemic, reflecting the unique and still controversially interpreted processes that triggered the emergence of the formation (Gross, 1977; Burg et al., 1992; Fleurance et al., 2013; Sokol et al., 2007, 2010, 2012; Novikov et al., 2013; Britvin et al., 2021a, 2022b, c, d). In this paper we focus on a specific subset of phosphate minerals, pure oxyphosphates, which are characterized by (1) an anhydrous composition, (2) the presence of a so-called “additional” oxygen atom in their chemical formulas and (3) the absence of other anions. These oxyphosphates are now comprised by six terrestrial species and two minerals of meteoritic origin (Table 1). We herein present a description of moabite, NiFe3+(PO4)O, a new representative of natural oxyphosphates. The mineral is named for the Kingdom of Moab, an ancient state that emerged in the late 10th century to 11th century BCE on the territory of the modern Hashemite Kingdom of Jordan (e.g. Finkelstein and Lipschits, 2011). The holotype specimen of moabite is deposited in the collections of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia, with the registration number 5627/1.
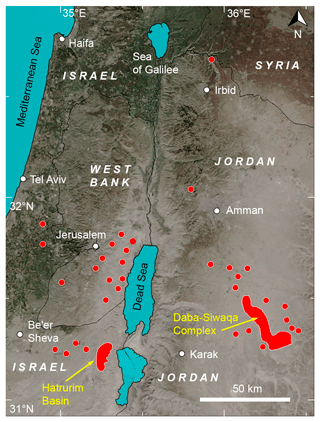
Figure 1Location of the Hatrurim Formation outcrops across the territory of the southern Levant. The largest massif is a Daba-Siwaqa complex in Jordan. Adapted from Britvin et al. (2021d) (CC-BY license).
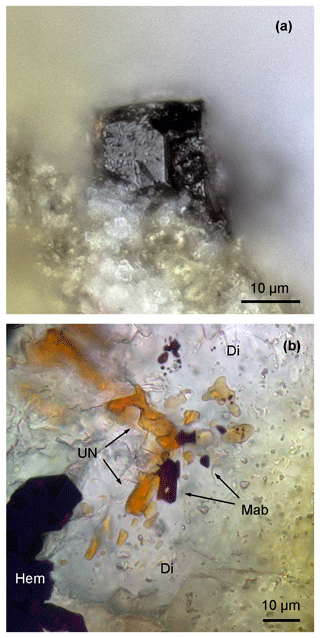
Figure 2(a) Moabite crystal in an anorthite–diopside matrix. (b) Thin section of the paralava containing moabite (Mab), unnamed alluaudite-group phosphate (UN) and hematite (Hem) in diopside matrix (Di). Transmitted light, parallel polars.
Electron microprobe analysis was conducted on the polished carbon-coated thin sections, using a WAVE 500 wavelength-dispersive spectrometer (Oxford Instruments) attached to a Hitachi S-3400 N scanning electron microscope. The spectrometer was operated at an acceleration voltage of 20 kV, beam current of 15 nA and beam diameter of 2 µm. The following standards and Kα lines were used: diopside (Mg), trevorite (Ni), Cu metal (Cu), hematite (Fe), gehlenite (Al), V metal (V), Cr metal (Cr) and chlorapatite (P). The same polished sections were used for subsequent spectroscopic studies after the removal of carbon film. Optical reflectance data were acquired by means of a Leica DM4500P microscope and TIDAS E MSP spectrophotometer calibrated against the Si standard.
The Raman spectrum was obtained by means of a LabRAM HR800 (HORIBA Jobin Yvon) spectrometer equipped with an Olympus microscope, 50× confocal objective and He–Ne laser (λ= 632.8 nm). X-ray single-crystal data were collected using a Bruker Kappa APEX DUO Diffractometer (CCD detector, charge-coupled device), equipped with a microfocus tube (MoKα radiation). The crystal structure was solved and refined using the SHELX-2018 set of programs (Sheldrick, 2015) embedded into the Olex2 graphical user interface (Dolomanov et al., 2009). Data collection and structure refinement details are summarized in the section on crystal structure. Full crystallographic information can be retrieved from the crystallographic information file (CIF) attached to the Supplement. X-ray powder diffraction data were obtained by means of a Rigaku RAXIS Rapid II diffractometer (semi-cylindrical imaging plate, Debye–Scherrer geometry, r= 127.4 mm), under the following conditions: CoKα radiation (rotating anode with microfocus tube), 40 kV, 15 mA and 60 min exposure. Conversion of the image plate to the profile and processing were carried out using the ocs2xrd program (Britvin et al., 2017) and Stoe WinXPOW v2.03 (Stoe & Cie GmbH, Darmstadt, Germany).
The geological setting of the Hatrurim Formation is described in numerous reports and reviews (Gross, 1977; Burg et al., 1992; Fleurance et al., 2013; Novikov et al., 2013; Abzalov et al., 2015; Sokol et al., 2019; Vereshchagin et al., 2024b). In brief, the formation, also known as “the Mottled Zone”, is comprised of dozens of rock outcrops exposed on both the Jordanian and Israeli sides of the Dead Sea, with some patches traced far north along the Jordan River (Fig. 1). The spread of the Mottled Zone outcrops coincides with the area affected by the tectonics of the Dead Sea transform fault system (Ben-Avraham et al., 2008). The age dating of the pyrometamorphic event(s) gives controversial results, from 16 Ma to even ∼ 250 ka (e.g. Sokol et al., 2019). Thermal metamorphic processes, initiated and maintained by one or more as yet poorly defined heat sources, resulted in temperatures up to 1400 °C at a near-atmospheric pressure (e.g. Vapnik et al., 2007). These P–T (pressure–temperature) conditions led to calcination and even fusion of sedimentary strata – chalks, marls and limestones – to form a variety of metamorphic lithologies, from marbles to the so-called “paralavas” (Vapnik et al., 2007). The 300 km2 Daba-Siwaqa complex in central Jordan is a largest representative of the Hatrurim Formation (Abzalov et al., 2015; Vereshchagin et al., 2024b). The numerous abandoned quarries formerly operated for building stone expose the whole palette of fresh pyrometamorphic rocks. Moabite-bearing assemblages were found in a small quarry (31°21′52′′ N, 36°10′55′′ E), within the blocks of veined anorthite–diopside paralava, whose assemblages were previously described in detail (Britvin et al., 2023a; Vereshchagin et al., 2024b). Moabite is an accessory phase in these assemblages, where it associates with diopside; anorthite; native iron; hematite; and a series of recently discovered phosphides and phosphates – negevite, NiP2; murashkoite, FeP; transjordanite, Ni2P; halamishite, Ni5P4; crocobelonite, CaFe(PO4)2O; yakubovichite, CaNi2Fe3+(PO4)3; and an alluaudite-group phosphate whose composition is exactly midway between the two endmembers NaNaCa(Fe3+Mg)(PO4)3 and □NaCa(Fe3+Fe3+)(PO4)3. The relationships between the minerals indicate that moabite is the earliest phosphate but formed after hematite, iron and phosphide minerals.
Moabite forms isometric to short prismatic crystals (Fig. 2a) and anhedral grains (Fig. 2b) up to 30 µm in size disseminated in a fine-grained matrix of anorthite–diopside paralava. The mineral often intergrows with other phosphates (Fig. 1b). Macroscopically, moabite has very dark-brown, almost-black, colour with vitreous lustre. In the thin sections in transmitted light, the mineral is semi-transparent with a red-brown colour. Due to the very high predicted value of the mean refractive index, 1.99, determination of refractive indices in transmitted light was considered impractical. In reflected light, the mineral has a grey colour with red-brown internal reflections. It is non-pleochroic, with weak anisotropy and bireflectance. Reflectance values are presented in Table 2.
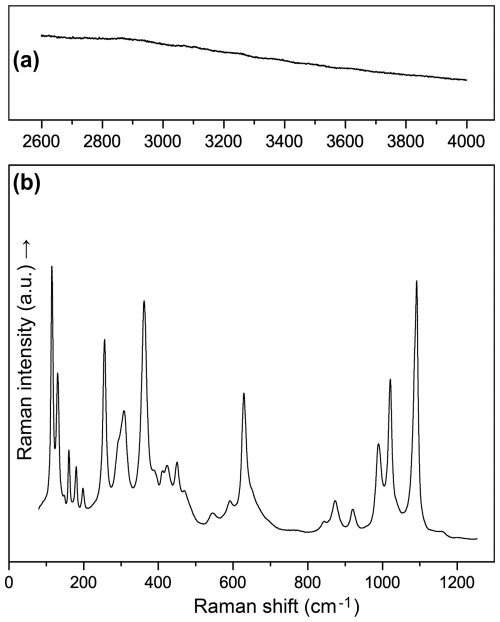
The Raman spectrum of moabite (Fig. 3) contains the following bands (cm−1): 115, 130, 160, 179, 198, 255, 292sh, 307, 361 and 388 (internal vibrations of [MO6] octahedra and lattice modes); 410, 423, 449 and 469 [ν2 (symmetric bending vibrations of (PO4) tetrahedra)]; 545, 590, 628 and 650sh [ν4 (asymmetric bending vibrations of (PO4) tetrahedra)]; 843, 871 and 918 [ν1 (symmetric stretching modes of P–O bonds)]; and 988, 1019, 1090 and 1157 [ν3 (asymmetric stretching vibrations of P–O)]. The band assignments were made based on the data previously reported for α-Fe2PO5 (Wu et al., 2018) and CoIn(PO4)O (El Arni et al., 2023). The absence of bands in the region of stretching vibrations of O–H bonds (3800–2800 cm−1) and bending modes of H2O at 1600–1650 cm−1 provides evidence of the absence of water in the chemical composition of moabite.
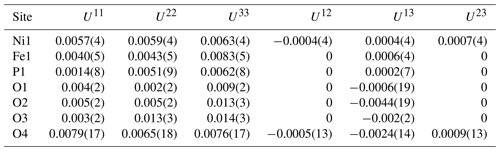
Moabite crystals and grains have rather uniform chemical composition, with no zoning. Among the non-essential elements substituting for Ni and Fe, one can mention Cu, which was not previously detected in phosphates of the Hatrurim Formation. The Raman spectrum, the results of the structural study and bond-valence calculations are consistent with the absence of water in the composition of the mineral. Consequently, the average results of microprobe analyses (Table 3) can be calculated on the basis of 5 oxygen atoms per formula unit (apfu), yielding the empirical formula (Ni0.90Cu0.05Mg0.03)Σ0.98(FeAl0.01Cr0.01)Σ1.03 (P0.99V)Σ1.00O5, which corresponds to the ideal NiFe3+(PO4)O.
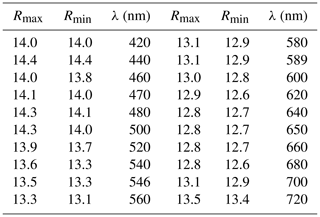
Indexing of X-ray powder diffraction data for moabite (Table 4) and refinement of the unit-cell parameters (orthorhombic system, space group Pnma) give the following results: a= 7.2145(3), b= 6.4031(3), c= 7.4657(3) Å and V= 344.88(2) Å3. These parameters are consistent with the results obtained by the single-crystal study, which are provided in Table 5 along with the basic parameters of single-crystal data collection and structure refinement. Fractional atomic coordinates and displacement parameters for moabite are presented in Tables 6 and 7.
Table 4X-ray powder diffraction data for moabite.
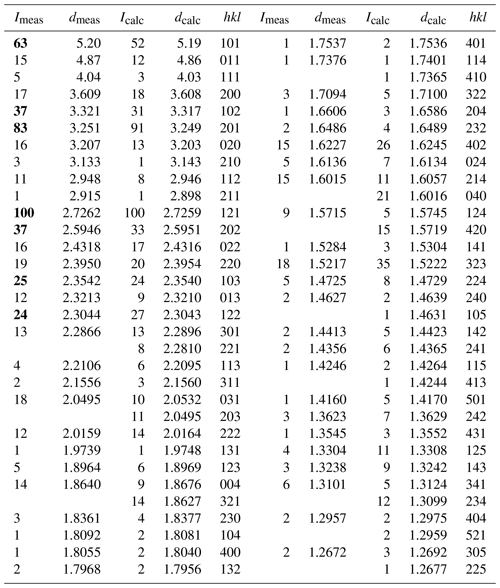
The measured intensities of the seven strongest lines are highlighted in bold. The theoretical pattern was calculated on the basis of atomic coordinates obtained from structure refinement and unit-cell parameters refined from powder data. Calculated lines with an intensity of less than 1 have been omitted.
Moabite has a synthetic analogue, NiFe3+(PO4)O (El Khayati et al., 2001); both crystallize in the α-Fe2PO5 structure type (Modaressi et al., 1983; Chemseddine and El Hajbi, 1999). The mineral and synthetic compounds exhibit almost identical bond-valence sums (Table 8), providing evidence for the proper assignment of the metal oxidation states in moabite. Moabite is the first mineral that adopts the α-Fe2PO5 structure type (Table 9). Synthetic orthorhombic α-Fe2PO5 ≡ Fe2+Fe3+(PO4)O, the structural prototype and Fe2+ analogue of moabite, is a high-temperature polymorph of Fe2PO5. Its low-temperature modification, β-Fe2PO5, adopts monoclinic (distorted-tetragonal) symmetry (Ech-Chahed et al., 1988; Ijjaali et al., 1990; Elkaïm et al., 1996). The crystal structure of the mineral consists of zigzag chains of alternating octahedra of [NiO6] and [FeO6] connected via the common faces, where [FeO6] octahedra are located at the bends of the chains (Fig. 4). The oxide chains, propagated along the b axis, are linked both through the external corners of the [FeO6] octahedra and via the corners of the [PO4] tetrahedra.
Moabite is isotypic to transition metal oxyphosphates of the general formula (PO4)O [OPO4], where Fe, Ni, Co and Cu and Fe, V and In, which are extensively studied due to their magnetic and electrochemical properties (Ech-Chahed et al., 1988; El Khayati et al., 2001; Aziam et al., 2018, 2020; El Arni et al., 2023). There are two known oxyphosphate minerals with the stoichiometry M2PO5: staněkite, Mn2+Fe3+(PO4)O (Keller et al., 1997), and joosteite, Mn2+Mn3+(PO4)O (Keller et al., 2007a) (Table 9). These minerals have crystal structures different from moabite and synthetic M2PO5 oxyphosphates.
Table 6Fractional atomic coordinates and isotropic displacement parameters (Uiso, Å2) for moabite.
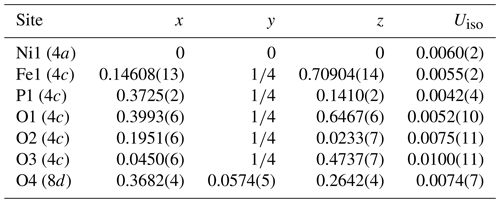
Note: Crystallographic tables were created using the PublCIF software (Westrip, 2010). Site multiplicities and Wyckoff symbols are given in parentheses.
Table 8Selected bond length (Å) and bond-valence sum (BVS; v.u., valence unit) values for cationic sites of moabite and synthetic NiFe(PO4)O*.
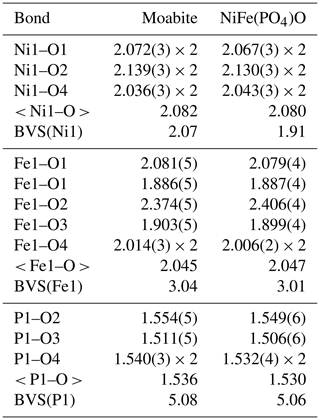
* Bond-valence sums for moabite were calculated using microprobe-determined site populations normalized to 1, and bond-valence parameters were derived by Gagné and Hawthorne (2015). Bond-valence sums for synthetic NiFe(PO4)O were taken from El Khayati et al. (2001).
Moabite, which was discovered in pyrometamorphic assemblages, is a product of high-temperature pyrolitic oxidation of natural iron–nickel phosphides, as shown for crocobelonite, CaFe(PO4)2O (Britvin et al., 2023a). Phosphide assemblages are the typical feature of the Hatrurim Formation; they are known from several localities across Israel, Palestine and Jordan (Britvin et al., 2015, 2020a; Galuskin et al., 2023a). It is noteworthy that rare phosphide findings were also reported from other localities of pyrometamorphic origin (Savina et al., 2020). In addition, natural phosphides occur in terrestrial basaltic iron assemblages (Klöck et al., 1986; Ulff-Møller, 1990; Vereshchagin et al., 2024a); they were found in coal-burnt dumps (Nishanbaev et al., 2002), mantle lithologies (Yang et al., 2005), fulgurites (Pasek and Block, 2009; Plyashkevich et al., 2016) and seabed sediments (Borodaev et al., 1982). Not all of these occurrences are suitable for postformational oxidation of phosphides, yet pyrometamorphism appears to present the most variable redox conditions. Phosphides, such as murashkoite, FeP; transjordanite–barringerite series, Ni2P-Fe2P; zuktamrurite, FeP2; negevite, NiP2; schreibersite, (Fe,Ni)3P; and others (Britvin et al., 2020c; Galuskin et al., 2023a), are frequently associated with native iron, forming a fruitful basement for high-temperature oxidative transformations. In the case of oxidation alone, pure Fe–Ni phosphates are being formed, according to e.g. the following scheme: 2(FeNi)P (barringerite–transjordanite) + 5O2→ 2FeNi(PO4)O (moabite).
In general, however, side reactions with the surrounding Ca-bearing silicates do occur, resulting in formation of more common Ca–Fe-bearing phosphates and oxyphosphates (Britvin et al., 2023a). The oxidation state of Ni in phosphates reaches Ni2+, whereas Fe state may vary between Fe2+ and Fe3+, depending on the local redox environment (Britvin et al., 2021d, 2023a).
Moabite, a Fe–Ni oxyphosphate, is a potential candidate for the occurrence in iron and stony-iron meteorites, where it could be formed by the oxidation of Fe–Ni metal–phosphide assemblages during high-temperature ablation processes. The recent discovery of ablation-formed iron oxyphosphates in the El Ali iron meteorite (Herd et al., 2024) shows that ablation-formed phosphates deserve further investigation, with the possibility of the recognition of new mineral phases.
The CIF file is available in the Supplement.
The supplement related to this article is available online at https://doi.org/10.5194/ejm-37-353-2025-supplement.
MNM and YV found the specimens containing the mineral. SNB recognized the mineral in the samples, calculated the solution and performed the refinement of its crystal structure, interpreted the Raman spectrum, and wrote the manuscript. MGK processed X-ray powder diffraction data. NSV and OSV carried out electron microprobe analyses. DVP obtained the Raman spectrum. EAV collected the optical reflectance spectrum.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors thank Ian Grey and the anonymous reviewer for their constructive comments and suggestions that improved the quality of the paper. The studies were carried out with instrumental and computational support of the Resource Centre for X-ray Diffraction Studies and Centre for Geo-Environmental Research and Modelling (GEOMODEL) of Saint Petersburg State University.
This research has been supported by the Russian Science Foundation (grant no. 23-77-10025).
This paper was edited by Sergey Krivovichev and reviewed by Ian Grey and one anonymous referee.
Abzalov, M. Z., Van Der Heyden, A., Saymeh, A., and Abuqudaira, M.: Geology and metallogeny of Jordanian uranium deposits, Appl. Earth Sci. Trans. Inst. Min., 124, 63–77, https://doi.org/10.1179/1743275815Y.0000000009, 2015.
Aziam, H., Garhi, G., Tamraoui, Y., Ma, L., Wu, T., Xu, G. L., Manoun, B., Alami, J., Amine, K., and Saadoune, I.: Understanding the electrochemical lithiation delithiation process in the anode material for lithium ion batteries NiFeOPO4 C using ex-situ X-ray absorption near edge spectroscopy and in-situ synchrotron X-ray, Electrochim. Ac., 283, 1238–1244, https://doi.org/10.1016/j.electacta.2018.07.038, 2018.
Aziam, H., Youcef, H. B., and Saadoune, I.: Evidence of CoFeOPO4 activity in Na-ion batteries, J. Electroanal. Chem., 874, 114450, https://doi.org/10.1016/j.jelechem.2020.114450, 2020.
Ben-Avraham, Z., Garfunkel, Z., and Lazar, M.: Geology and evolution of the Southern Dead Sea Fault with emphasis on subsurface structure, Ann. Rev. Earth Planet. Sci., 36, 357–387, https://doi.org/10.1146/annurev.earth.36.031207.124201, 2008.
Borodaev, Y. S., Bogdanov, Y. A., and Vyalsov, L. N.: New nickel-free variety of schreibersite Fe3P, Proc. All-Union Mineral. Soc., 111, 682–687, 1982 (in Russian).
Britvin, S. N., Murashko, M. N., Vapnik, Y., Polekhovsky, Y. S., and Krivovichev, S. V.: Earth's Phosphides in Levant and insights into the source of Archean prebiotic phosphorus, Sci. Rep., 5, 8355, https://doi.org/10.1038/srep08355, 2015.
Britvin, S. N., Dolivo-Dobrovolsky, D. V., and Krzhizhanovskaya, M. G.: Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Mineral. Obshch., 146, 104–107, 2017 (in Russian).
Britvin, S. N., Murashko, M. N., Vapnik, Ye., Polekhovsky, Yu. S., Krivovichev, S. V., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Shilovskikh, V. V., and Vlasenko, N. S.: Transjordanite, Ni2P, a new terrestrial and meteoritic phosphide, and natural solid solutions barringerite–transjordanite (hexagonal Fe2P–Ni2P). Am. Mineral., 105, 428–436, https://doi.org/10.2138/am-2020-7275, 2020a.
Britvin, S. N., Murashko, M. N., Vapnik, Ye., Polekhovsky, Yu. S., Krivovichev, S. V., Vereshchagin, O. S., Shilovskikh, V. V., Vlasenko, N. S., and Krzhizhanovskaya, M. G.: Halamishite, Ni5P4, a new terrestrial phosphide in the Ni-P system, Phys. Chem. Miner., 47, 3, https://doi.org/10.1007/s00269-019-01073-7, 2020b.
Britvin, S. N., Murashko, M. N., Vapnik, Ye., Polekhovsky, Yu. S., Krivovichev, S. V., Vereshchagin, O. S., Shilovskikh, V. V., and Krzhizhanovskaya, M. G.: Negevite, the pyrite-type NiP2, a new terrestrial phosphide, Am. Mineral., 105, 422–427, https://doi.org/10.2138/am-2020-7192, 2020c.
Britvin, S. N., Vereshchagin, O. S., Shilovskikh, V. V., Krzhizhanovskaya, M. G., Gorelova, L. A., Vlasenko, N. S., Pakhomova, A. S., Zaitsev, A. N., Zolotarev, A. A., Bykov, M., Lozhkin, M. S., and Nestola, F.: Discovery of terrestrial allabogdanite (Fe, Ni)2P, and the effect of Ni and Mo substitution on the barringerite-allabogdanite high-pressure transition, Am. Mineral., 106, 944–952, https://doi.org/10.2138/am-2021-7621, 2021a.
Britvin, S. N., Krzhizhanovskaya, M. G., Zolotarev, A. A., Gorelova, L. A., Obolonskaya, E. V., Vlasenko, N. S., Shilovskikh, V. V., and Murashko, M. N.: Crystal chemistry of schreibersite, (Fe, Ni)3P, Am. Mineral., 106, 1520–1529, https://doi.org/10.2138/am-2021-7766, 2021b.
Britvin, S. N., Galuskina, I. O., Vlasenko, N. S., Vereshchagin, O. S., Bocharov, V. N., Krzhizhanovskaya, M. G., Shilovskikh, V. V., Galuskin, E. V., Vapnik, Y., and Obolonskaya, E. V.: Keplerite, Ca9(Ca0.5□0.5)Mg(PO4)7, a new meteoritic and terrestrial phosphate isomorphous with merrillite, Ca9NaMg(PO4)7, Am. Mineral, 106, 1917–1927, https://doi.org/10.2138/am-2021-7834, 2021c.
Britvin, S. N., Murashko, M. N., Vapnik, Ye., Vlasenko, N. S., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Bocharov, V. N., and Lozhkin, M. S.: Cyclophosphates, a new class of native phosphorus compounds, and some insights into prebiotic phosphorylation on early Earth, Geology, 49, 382–386, https://doi.org/10.1130/G48203.1, 2021d.
Britvin, S. N., Murashko, M. N., Vapnik, Ye., Zaitsev, A. N., Shilovskikh, V. V., Krzhizhanovskaya, M. G., Gorelova, L. A., Vereshchagin, O. S., Vasilev, E. A., and Vlasenko, N. S.: Orishchinite, a new terrestrial phosphide, the Ni-dominant analogue of allabogdanite, Mineral. Petrol., 116, 369–378, https://doi.org/10.1007/s00710-022-00787-x, 2022a.
Britvin, S. N., Vlasenko, N. S., Aslandukov, A., Aslandukova, A., Dubrovinsky, L., Gorelova, L. A., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Bocharov, V. N., Shelukhina, Yu. S., Lozhkin, M. S., Zaitsev, A. N., and Nestola, F.: Natural cubic perovskite, Ca(Ti,Si,Cr)O3−δ, a versatile potential host for rock-forming and less common elements up to Earth's mantle pressure, Am. Mineral., 107, 1936–1945, https://doi.org/10.2138/am-2022-8186, 2022b.
Britvin, S. N., Murashko, M. N., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Vapnik, Ye., Shilovskikh, V. V., Lozhkin, M. S., and Obolonskaya, E. V.: Nazarovite, Ni12P5, a new terrestrial and meteoritic mineral structurally related to nickelphosphide, Ni3P, Am. Mineral., 107, 1946–1951, https://doi.org/10.2138/am-2022-8219, 2022c.
Britvin, S. N., Murashko, M. N., Vereshchagin, O. S., Vapnik, Ye., Shilovskikh, V. V., Vlasenko, N. S., and Permyakov, V. V.: Expanding the speciation of terrestrial molybdenum: Discovery of polekhovskyite, MoNiP2, and insights into the sources of Mo-phosphides in the Dead Sea Transform area, Am. Mineral., 107, 2201–2211, https://doi.org/10.2138/am-2022-8261, 2022d.
Britvin, S. N., Murashko, M. N., Krzhizhanovskaya, M. G., Vlasenko, N. S., Vereshchagin, O. S., Vapnik, Y., and Bocharov, V. N.: Crocobelonite, CaFe(PO4)2O, a new oxyphosphate mineral, the product of pyrolytic oxidation of natural phosphides, Am. Mineral., 108, 1973–1983, https://doi.org/10.2138/am-2022-8757, 2023a.
Britvin, S. N., Murashko, M. N., Krzhizhanovskaya, M. G., Vapnik, Ye., Vlasenko, N. S., Vereshchagin, O. S., Pankin, D. V., Zaitsev, A. N., and Zolotarev, A. A.: Yakubovichite, CaNi2Fe3+(PO4)3, a new nickel phosphate mineral of non-meteoritic origin, Am. Mineral., 108, 2142–2150, https://doi.org/10.2138/am-2022-8800, 2023b.
Burg, A., Starinsky, A., Bartov, Y., and Kolodny, Y.: Geology of the Hatrurim Formation (“Mottled Zone”) in the Hatrurim basin, Isr. J. Earth Sci., 40, 107–124, 1992.
Chemseddine, E. and El Hajbi, A.: Application of the alternating spin chain model to the α-Fe2PO5 compound, Ann. Chim. Sci. Matér., 24, 241–244, https://doi.org/10.1016/S0151-9107(99)80050-6, 1999.
Cipriani, C., Mellini, M., Pratesi, G., and Viti, C.: Rodolicoite and grattarolaite, two new phosphate minerals from Santa Barbara Mine, Italy, Eur. J. Mineral., 9, 1101–1106, https://doi.org/10.1127/ejm/9/5/1101, 1997.
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A., and Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Cryst., 42, 339–341, https://doi.org/10.1107/S0021889808042726, 2009.
Ech-Chahed, B., Jeannot, F., Malaman, B., and Gleitzer, C.: Préparation etétude d'une variétébasse température de l'oxyphosphate de fer de valence mixte βFe2(PO4)O et de NiCr(PO4)O: Un cas d'échangeélectronique rapide, J. Solid State Chem., 74, 47–59, https://doi.org/10.1016/0022-4596(88)90330-1, 1988.
Elkaïm, E., Berar, J. F., Gleitzer, C., Malaman, B., Ijjaali, M., and Lecomte, C.: Occurrence of a monoclinic distortion in β-Fe2PO5, Acta Crystallogr. B, 52, 428–431, https://doi.org/10.1107/S0108768195014273, 1996.
El Arni, S., Rguig, O., Hadouchi, M., Assani, A., Saadi, M., Lahmar, A., Bouyanfif, H., El Marssi, M., and El Ammari, L.: Synthesis, structural characterization and magnetic properties of CoInOPO4 with the α-Fe2OPO4 structure, J. Solid State Chem., 324, 124111, https://doi.org/10.1016/j.jssc.2023.124111, 2023.
El Khayati, N., Cherkaoui El Moursli, R., Rodríguez-Carvajal, J., André, G., Blanchard, N., Bourée, F., Collin, G., and Roisnel, T.: Crystal and magnetic structures of the oxophosphates MFePO5 (M= Fe, Co, Ni, Cu), Analysis of the magnetic ground state in terms of superexchange interactions, Eur. Phys. J. B, 22, 429–442, https://doi.org/10.1007/s100510170093, 2001.
Finkelstein, I. and Lipschits, O.: The genesis of Moab: a proposal, Levant, 43, 139–152, https://doi.org/10.1179/175638011X13112549593005, 2011.
Fleurance, S., Cuney, M., Malartre, M., and Reyx, J.: Origin of the extreme polymetallic enrichment (Cd, Cr, Mo, Ni, U, V, Zn) of the Late Cretaceous–Early Tertiary Belqa Group, central Jordan, Palaeogeogr. Palaeocl., 369, 201–219, https://doi.org/10.1016/j.palaeo.2012.10.020, 2013.
Gagné, O. C. and Hawthorne, F. C.: Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen, Acta Crystallogr. B, 71, 562–578, https://doi.org/10.1107/S2052520615016297, 2015.
Galuskin, E. V., Gfeller, F., Galuskina, I. O., Pakhomova, A., Armbruster, T., Vapnik, Y., Włodyka, R., Dzierżanowski, P., and Murashko, M.: New minerals with a modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex, Part II, Zadovite, BaCa6[(SiO4)(PO4)](PO4)2F and aradite, BaCa6[(SiO4)(VO4)](VO4)2F, from paralavas of the Hatrurim Basin, Mineral. Mag., 79, 1073–1087, https://doi.org/10.1180/minmag.2015.079.5.04, 2015.
Galuskin, E. V., Galuskina, I. O., Gfeller, F., Krüger, B., Kusz, J., Vapnik, Y., Dulski, M., and Dzierżanowski, P.: Silicocarnotite, Ca5[(SiO4)(PO4)](PO4), a new ”old” mineral from the Negev Desert, Israel, and the ternesite–silicocarnotite solid solution: indicators of high-temperature alteration of pyrometamorphic rocks of the Hatrurim Complex, Southern Levant, Eur. J. Mineral., 28, 105–123, https://doi.org/10.1127/ejm/2015/0027-2494, 2016.
Galuskin, E. V., Krüger, B., Galuskina, I. O., Krüger, H., Vapnik, Y., Pauluhn, A., and Olieric, V.: Stracherite, BaCa6(SiO4)2[(PO4)(CO3)]F, the first CO3-bearing intercalated hexagonal antiperovskite from Negev Desert, Israel, Am. Mineral., 103, 1699–1706, https://doi.org/10.2138/am-2018-6493, 2018a.
Galuskin, E. V., Krüger, B., Galuskina, I. O., Krüger, H., Vapnik, Y., Wojdyla, J. A., and Murashko, M.: New mineral with modular structure derived from hatrurite from the pyrometamorphic rocks of the Hatrurim Complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev Desert, Israel, Minerals, 8, 109, https://doi.org/10.3390/min8030109, 2018b.
Galuskin, E. V., Krüger, B., Galuskina, I. O., Krüger, H., Vapnik, Y., Pauluhn, A., and Olieric, V.: Levantite, KCa3(Al2Si3O11(PO4), a new latiumite-group mineral from the pyrometamorphic rocks of the Hatrurim Basin, Negev Desert, Israel, Mineral. Mag., 83, 713–721, https://doi.org/10.1180/mgm.2019.37, 2019.
Galuskin, E., Galuskina, I., Krüger, B., Krüger, H., Vapnik, Y., Krzątała, A., Środek, D., and Zieliński, G.: Nomenclature and Classification of the Arctite Supergroup, Aravaite, Ba2Ca18(SiO4)6(PO4)3(CO3)F3O, a New Arctite Supergroup Mineral from Negev Desert, Israel, Can. Mineral., 59, 191–209, https://doi.org/10.3749/canmin.2000035, 2021.
Galuskin, E. V., Stachowicz, M., Galuskina, I. O., Wózniak, K., Vapnik, Y., Murashko, M. N., and Zielínski, G.: Deynekoite, Ca9Fe3+(PO4)7 – a new mineral of the merrillite group from phosphide-bearing contact facies of paralava, Hatrurim Complex, Daba-Siwaqa, Jordan, Mineral. Mag., 87, 943–954, https://doi.org/10.1180/mgm.2023.71, 2023a.
Galuskin, E. V., Kusz, J., Galuskina, I. O., Książek, M., Vapnik, Ye., and Zieliński, G.: Discovery of terrestrial andreyivanovite, FeCrP, and the effect of Cr and V substitution on the low-pressure barringerite-allabogdanite transition, Am. Mineral., 108, 1506–1515, https://doi.org/10.2138/am-2022-8647, 2023b.
Galuskin, E. V., Galuskina, I. O., Kusz, J., Książek, M., Vapnik, Y., and Zieliński, G.: Karwowskiite, Ca9(Fe)Mg(PO4)7 – A New Merrillite Group Mineral from Paralava of the Hatrurim Complex, Daba-Siwaqa, Jordan, Minerals, 14, 825, https://doi.org/10.3390/min14080825, 2024.
Gross, S.: The mineralogy of the Hatrurim Formation, Israel, Geol. Surv. Isr. Bull., 70, 1–80, 1977.
Herd, C. D. K., Ma, C., Locock, A. J., Saini, R., and Walton, E. L.: Three new iron-phosphate minerals from the El Ali iron meteorite, Somalia: Elaliite FeFe3+(PO4)O8, elkinstantonite Fe4(PO4)2O, and olsenite KFe4(PO4)3, Am. Mineral., 109, 2142–2151, https://doi.org/10.2138/am-2023-9225, 2024.
Ijjaali, M., Malaman, B., Gleitzer, C., Warner, J. K., Hriljac, J. A., and Cheetham, K. A.: Stability, structure refinement, and magnetic properties of β-Fe2(PO4)O, J. Solid State Chem., 86, 195–205, https://doi.org/10.1016/0022-4596(90)90135-K, 1990.
Juroszek, R., Galuskina, I., Krüger, B., Krüger, H., Vapnik, Ye., Kahlenberg, V., and Galuskin, E.: Minerals with a palmierite-type structure. Part I. Mazorite Ba3(PO4)2, a new mineral from the Hatrurim Complex in Israel, Mineral. Mag., 87, 679–689, https://doi.org/10.1180/mgm.2023.57, 2023.
Keller, P., Fontan, F., Velasco-Roldan, F., and Melgarejo i Draper, J. C.: Staněkite, Fe3+(Mn,Fe2+, Mg)(PO4)O: a new phosphate mineral in pegmatites at Karibib (Namibia) and French Pyrenees (France), Eur. J. Mineral., 9, 475–482, https://doi.org/10.1127/ejm/9/3/0475, 1997.
Keller, P., Lissner, F., and Schleid, T.: The crystal structure of staněkite, (Fe3+, Mn2+, Fe2+,Mg)2[PO4]O, from Okatjimukuju, Karibib (Namibia), and its relationship to the polymorphs of synthetic Fe2[PO4]O, Eur. J. Mineral., 18, 113–118, https://doi.org/10.1127/0935-1221/2006/0018-0113, 2006.
Keller, P., Fontan, F., Roldan, F. V., and de Parseval, P.: Joosteite, Mn2+(Mn3+, Fe3+)(PO4)O: a new phosphate mineral from the Helikon II Mine, Karibib, Namibia, N. Jahrb. Mineral. Abh., 183, 197–201, 10.1127/0077-7757/2007/0069, 2007a.
Keller, P., Lissner, F., and Schleid, T.: The crystal structure of joosteite, (Mn2+, Mn3+, Fe3+)2(PO4)O, from the Helikon II Mine, Karibib (Namibia) and its relationship to stanekite, (Fe3+, Mn2+, Fe2+, Mg)2(PO4)O, N. Jahrb. Mineral. Abh., 184, 225–230, https://doi.org/10.1127/0077-7757/2007/0095, 2007b.
Klöck, W., Palme, H., and Tobschall, H. J.: Trace elements in natural metallic iron from Disko Island, Greenland, Contrib. Mineral. Petrol., 93, 273–282, https://doi.org/10.1007/BF00389387, 1986.
Krzątała, A., Skrzyńska, K., Cametti, G., Galuskina, I., Vapnik, Ye., and Galuskin, E.: Fluoralforsite, Ba5(PO4)3F – a new apatite-group mineral from the Hatrurim Basin, Negev Desert, Israel, Mineral. Mag., 87, 866–877, https://doi.org/10.1180/mgm.2023.58, 2023.
Modaressi, A., Courtois, A., Gerardin, R., Malaman, B., and Gleitzer, C.: Fe3PO7, Un cas de coordinence 5 du fer trivalent, etude structurale et magnetique, J. Solid State Chem., 47, 245–255, https://doi.org/10.1016/0022-4596(83)90016-6, 1983.
Murashko, M. N., Britvin, S. N., Vapnik, Ye., Polekhovsky, Y. S., Shilovskikh, V. V., Zaitsev, A. N., and Vereshchagin, O. S.: Nickolayite, FeMoP, a new natural molybdenum phosphide, Mineral. Mag., 86, 749–757, https://doi.org/10.1180/mgm.2022.52, 2022.
Nishanbaev, T. P., Rochev, A. V., and Kotlyarov, V. A.: Iron phosphides from combustion dumps of Chelaybinsk coal basin, Ural. Geol. J., 25, 105–114, 2002 (in Russian).
Novikov, I., Vapnik, Ye., and Safonova, I.: Mud volcano origin of the Mottled Zone, Southern Levant, Geosci. Front., 4, 597–619, https://doi.org/10.1016/j.gsf.2013.02.005, 2013.
Pasek, M. and Block, K.: Lightning-induced reduction of phosphorus oxidation state, Nat. Geosci., 2, 553–556, https://doi.org/10.1038/ngeo580, 2009.
Plyashkevich, A. A., Minyuk, P. S., Subbotnikova, T. V., and Alshevsky, A. V.: Newly formed minerals of the Fe-P-S system in Kolymsky fulgurite, Dokl. Earth Sci., 467, 380–383, https://doi.org/10.1134/S1028334X16040139, 2016.
Savina, E. A., Peretyazhko, I. S., Khromova, E. A., and Glushkova, V. E.: Melted rocks (clinkers and paralavas) of Khamaryn-Khural-Khiid combustion metamorphic complex in Eastern Mongolia: Mineralogy, geochemistry and genesis, Petrology, 28, 431–457, https://doi.org/10.1134/S0869591120050057, 2020.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr. C, 71, 3–8, https://doi.org/10.1107/S2053229614024218, 2015.
Sokol, E. V., Novikov, I. S., Vapnik, Y., and Sharygin, V. V.: Gas fire from mud volcanoes as a trigger for the appearance of high-temperature pyrometamorphic rocks of the Hatrurim Formation (Dead Sea area), Dokl. Earth Sci., 413, 474–480, https://doi.org/10.1134/S1028334X07030348, 2007.
Sokol, E., Novikov, I., Zateeva, S., Vapnik, Ye., Shagam, R., and Kozmenko, O.: Combustion metamorphism in the Nabi Musa dome: New implications for a mud volcanic origin of the Mottled Zone, Dead Sea area, Basin Res., 22, 414–438, https://doi.org/10.1111/j.1365-2117.2010.00462.x, 2010.
Sokol, E. V., Kozmenko, O. A., Kokh, S. N., and Vapnik, Ye.: Gas reservoirs in the Dead Sea area: Evidence from chemistry of combustion metamorphic rocks in Nabi Musa fossil mud volcano, Russ. Geol. Geophys., 53, 745–762, https://doi.org/10.1016/j.rgg.2012.06.003, 2012.
Sokol, E. V., Seryotkin, Y. V., Kokh, S. N., Vapnik, Ye., Nigmatulina, E. N., Goryainov, S. V., Belogub, E. V., and Sharygin, V. V.: Flamite, (Ca,Na,K)2(Si,P)O4, a new mineral from ultrahigh-temperature combustion metamorphic rocks, Hatrurim Basin, Negev Desert, Israel, Mineral. Mag., 79, 583–596, https://doi.org/10.1180/minmag.2015.079.3.05, 2015.
Sokol, E. V., Kokh, S. N., Sharygin, V. V., Danilovsky, V. A., Seryotkin, Yu. V., Liferovich, R., Deviatiiarova, A. S., Nigmatulina, E. N., and Karmanov, N. S.: Mineralogical diversity of Ca2SiO4-bearing combustion metamorphic rocks in the Hatrurim Basin: Implications for storage and partitioning of elements in oil shale clinkering, Minerals, 9, 465, https://doi.org/10.3390/min9080465, 2019.
Ulff-Møller, F.: Formation of native iron in sediment-contaminated magma. I. A case study of the Hanekammen Complex on Disko Island, West Greenland, Geochim. Cosmochim. Ac., 54, 57–70, https://doi.org/10.1016/0016-7037(90)90195-Q, 1990.
Vapnik, Ye., Sharygin, V. V., Sokol, E. V., and Shagam, R.: Paralavas in a combustion metamorphic complex: Hatrurim Basin, Israel, Geol. Soc. Am. Rev. Eng. Geol., 18, 133–153, https://doi.org/10.1130/2007.4118(09), 2007.
Vereshchagin, O. S., Khmelnitskaya, M. O., Kamaeva, L. V., Vlasenko, N. S., Pankin, D. V., Bocharov, V. N., and Britvin, S. N.: Telluric iron assemblages as a source of prebiotic phosphorus on the early Earth: Insights from Disko Island, Greenland, Geosci. Front., 15, 101870, https://doi.org/10.1016/j.gsf.2024.101870, 2024a.
Vereshchagin, O. S., Khmelnitskaya, M. O., Murashko, M. N., Vapnik, Ye., Zaitsev, A. N., Vlasenko, N. S., Shilovskikh, V. V., and Britvin, S. N.: Reduced mineral assemblages of superficial origin in west-central Jordan, Mineral. Petrol., 118, 305–319, https://doi.org/10.1007/s00710-024-00851-8, 2024b.
Westrip, S. P.: publCIF: Software for Editing, Validating and Formatting Crystallographic Information Files, J. Appl. Cryst., 43, 920–925, https://doi.org/10.1107/S0021889810022120, 2010.
Wu, Y. Z., Meng, Y. N., Hou, J. G., Cao, S., Gao, Z., Wu, Z., and Sun, L.: Orienting active crystal planes of new class lacunaris Fe2PO5 polyhedrons for robust water oxidation in alkaline and neutral media, Adv. Funct. Mater., 28, 1801397, https://doi.org/10.1002/adfm.201801397, 2018.
Yang, J. S., Bai, W. J., Rong, H., Zhang, Z. M., Xu, Z. Q., Fang, Q. S., Yang, B. G., Li, T. F., Ren, Y. F., Chen, S. Y., Hu, J.-Z., Su, J. F., and Mao, H. K.: Discovery of Fe2P alloy in garnet peridotite from the Chinese continental scientific drilling project (CCSD) main hole, Acta Petrol. Sin., 21, 271–276, 2005.
- Abstract
- Introduction
- Samples and methods
- Occurrence
- Appearance and physical and optical properties
- Raman spectroscopy
- Chemical composition
- X-ray powder diffraction and crystal structure
- Discussion and conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Samples and methods
- Occurrence
- Appearance and physical and optical properties
- Raman spectroscopy
- Chemical composition
- X-ray powder diffraction and crystal structure
- Discussion and conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement