the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Nolanite supergroup of minerals: nomenclature and classification
Nikita V. Chukanov
Vasilisa M. Gridchina
Ramiza K. Rastsvetaeva
Natalia V. Zubkova
Igor V. Pekov
The nolanite supergroup has been established and approved by the IMA CNMNC. It contains eight mineral species with the nolanite-type structure. They are hexagonal with the space group P63mc and unit-cell parameters in the following ranges: a=5.5–6.0 Å and c=8.8–10.3 Å; Z=2. The nolanite supergroup is subdivided into three groups (nolanite, kamiokite, and rinmanite groups) in accordance with the largest charge of species-defining cations, which coincides with the largest charge of octahedral M cations (+3, +4, and +5, respectively). Their general formulae are M12O7(OH) (nolanite group: nolanite, VFe3+O7(OH); akdalaite, Al5O7(OH); and ferrihydrite, FeO7(OH)), M12O8 (kamiokite group: kamiokite, FeMoO8; iseite, MnMoO8; and majindeite, Mg2MoO8), and (M112+)M2O7(OH) (rinmanite group: rinmanite, (FeMg)Sb5+ZnO7(OH), and zincorinmanite-(Zn), (FeZn)Sb5+ZnO7(OH)). Relationships between members of each group can be described by homovalent substitution schemes, whereas relationships between different groups are determined only by heterovalent substitution schemes. All historical names of minerals belonging to the nolanite supergroup are preserved. In new minerals of the nolanite supergroup, each combination of the M1 and M2 cations defines the root name. A Levinson-type suffix should be applied to indicate the dominant component at the tetrahedrally coordinated T site. The charge-balancing M12+ cation defines the prefix (magnesio-, zinco-, mangano-, etc.).
- Article
(2816 KB) - Full-text XML
- BibTeX
- EndNote
Among oxide minerals with the stoichiometry M13M2TO7X (X= O, OH), there are eight mineral species with the nolanite-type structure (Fig. 1). All of them are hexagonal, with the space group P63mc and unit-cell parameters in the following ranges: a=5.5–6.0 Å and c=8.8–10.3 Å; Z=2. Their crystal structures are composed of alternating layers of two kinds: a layer of edge-sharing M1 octahedra (O layer) and a heteropolyhedral (H) layer of M2 octahedra and T tetrahedra connected via common vertices (Fig. 2). The M1 octahedra are connected with the H layers situated above and below it via common edges with M2 octahedra and via common vertices with T tetrahedra, and, thus, a quasi-framework forms. The X site is coordinated by M1 cations. The cations occupying polyhedra of the quasi-framework are very different in their charges. These minerals are different not only in bulk chemical composition but also in the distribution of cations over tetrahedral and octahedral sites (see Table 1 and references below). Nolanite, described in 1957 (Robinson et al., 1957), was the first mineral species belonging to this structure type.
Table 1Comparative data of nolanite-supergroup minerals with the general formula M13M2TO7X (X= O, OH).

Note: powder X-ray diffraction data for akdalaite and ferrihydrite published by Shpanov et al. (1971) and Chukhrov et al. (1973), respectively, may correspond to polluted samples. More reliable d values for the strongest lines of akdalaite (without data on their intensities) given by Hwang et al. (2006) are 4.84, 4.42, 4.23, 2.78, 2.53, 2.36, 1.67, 1.44, and 1.42 Å.
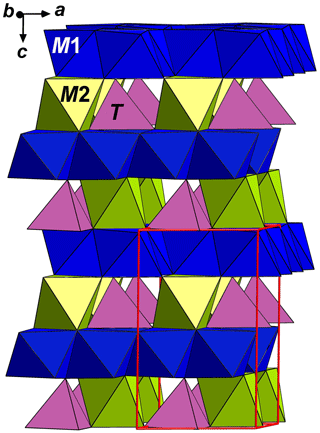
Figure 1The crystal structure of a nolanite-supergroup mineral: general view. The unit cell is outlined.
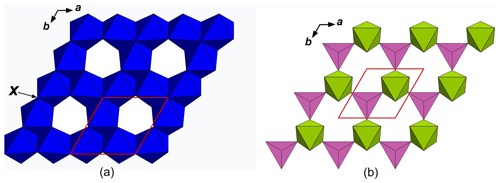
Figure 2Layer of octahedra (O layer) (a) and heteropolyhedral (H) layer (b) in the nolanite-type structure. The unit cell is outlined. For the legend, see Fig. 1.
The mineral species with the nolanite-type structure were united to a mineral supergroup named the nolanite supergroup. The nomenclature and classification of the nolanite supergroup presented in this paper were approved by the IMA Commission on New Minerals, Nomenclature and Classification in 2024 (Memorandum 127-SM24).
The guidelines for the identification of three groups within the nolanite supergroup are (1) the highest charge q1 of the chemical constituents at the octahedral M(1) and M(2) sites and (2) the dominant species at the X site (X= O or OH). In accordance with these criteria, the nolanite supergroup can be subdivided into three groups: nolanite group with (for both M1 and M2) and X= OH, kamiokite group with (for M1) and X= O, and rinmanite group with (for M2) and X= OH. The general formulae of minerals belonging to these groups are M12O7(OH), M12O8, and (M112+)M2O7(OH), respectively.
In this approach, octahedral positions are considered together. An additional criterion for the mineral group definition is the charge q2 of a cation in the tetrahedral T site: for the nolanite-group minerals and for minerals belonging to the kamiokite and rinmanite groups. This classification makes it possible to calculate crystal-chemical formulae of nolanite-supergroup minerals based on chemical data only (see below).
If compositions with and OH at X were to be found, that would constitute a new group. The general crystal-chemical formulae of the members of this group would be M12O7(OH) or (M112+)M2O7(OH) (the formula M12O7O is not allowed because the occurrence of R3+ at M1 site does not allow for such charge compensation at the X site).
Since the octahedral sites are considered together, minerals with the crystal chemical formulae (M112+)M2O7O and M12O7O are members of the same group (namely, kamiokite group) with the general formula RO8. This approach eliminates ambiguity in determining mineral species based on chemical composition data in the absence of structural data.
Relationships between members of each group can be described by homovalent substitution schemes, whereas relationships between different groups are determined only by heterovalent substitution schemes.
The nolanite group unites minerals containing only trivalent species-defining cations (). Their generalized crystal-chemical formula is M12O7(OH).
Nolanite, VFe3+O7(OH) = VV3+Fe3+O7(OH), has been discovered in a hydrothermal uranium deposit on the shore of Fish Hook Bay, Lake Athabasca, in the Beaverlodge region of Saskatchewan, Canada (Robinson et al., 1957). Thereafter, this mineral was found in gold deposits hosted by metamorphosed greenstones in Kalgoorlie, Western Australia (Taylor and Radtke, 1967; Gatehouse et al., 1983); in the Yushui Cu deposit, the province of Guangdong, South China (Liu et al., 2023); and in the Vihanti metamorphosed volcanic-hosted massive sulfide Zn deposit situated in the North Ostrobothnia region, Finland (Voloshin et al., 2014). Based on wet chemical analyses, it was initially supposed that nolanite contains significant amounts of Fe2+ and V4+ (along with V3+) and does not contain hydrogen (Robinson et al., 1957).
In the first model of the crystal structure of a sample from the type locality (Hanson, 1958), the site occupancies were not refined, and nolanite was tentatively considered a V4+-dominant mineral with the formula FeVV. However, subsequent chemical analyses and the refinement of the crystal structure of nolanite samples from Kalgoorlie showed that in this mineral, the tetrahedrally coordinated T site contains mainly Fe3+ (with subordinate Fe2+), and the M1 and M2 octahedra are occupied by V3+ with subordinate Ti and minor Al and/or Fe2+ (Gatehouse et al., 1983). The generalized crystal-chemical formula of nolanite from Kalgoorlie is M1(V3+,Ti,Al,Fe2+)3M2(VTi,Al,Fe2+)T(Fe3+,Fe2+) O7(OH). The total content of minor cations (Ti, Fe2+) involved in heterovalent substitutions in the studied samples is 0.3–0.55 atoms per formula unit (apfu) (Z=2).
Akdalaite, Al5O7(OH) = Al3AlAlO7(OH), has been discovered as imperfect elongate-tabular crystals up to 0.1×0.8 mm in veinlets composed of muscovite and fluorite at the Solnechnoe fluorite deposit, Karaganda Region, Kazakhstan (Shpanov et al., 1971). The bulk empirical formula of the holotype sample is (Al4.74Be0.09Mg0.07FeZn0.03)Σ4.97[O6.73(OH)1.21F0.06]Σ8.00, which is similar to the formulae of some members of the högbomite supergroup whose crystal structures contain a nolanite-type module. Regarding its physical, chemical, and powder X-ray diffraction characteristics, akdalaite is similar to the synthetic aluminum hydroxide, tohdite 5Al2O3•H2O, hexagonal, with a=5.575(1) Å and c=8.761(1) Å (Yamaguchi et al., 1964, 1969). However, in the first description of akdalaite (Shpanov et al., 1971) different unit-cell parameters are given: a=12.87 Å and c=14.97 Å. Subsequent studies have shown that these parameters are incorrect.
The crystal structure of tohdite has been refined using powder X-ray diffraction data with the Rietveld method (Yamaguchi et al., 1969). In this structure four-fifths of the aluminum atoms are in octahedral sites, and the rest are in tetrahedral sites. The refinement of the crystal structure of nolanite (Gatehouse et al., 1983) has confirmed that this mineral and tohdite, the synthetic akdalaite analogue, are isostructural.
Electron diffraction data for the akdalaite sample from the Kulet Kol region, Kokchetav Massif, northern Kazakhstan, yielded the unit-cell parameters a=5.58(1) Å and c=8.86(2) Å, space group P63mc (Hwang et al., 2006). The akdalaite-type specimen, re-examined by Hwang et al. (2006), has similar unit-cell parameters: a=5.59(1) Å and c=8.82(1) Å. Electron microprobe analyses of the sample from the Kokchetav Massif show minor Si, Ti, Cr, Fe, Mg, Zn, and Ga admixtures, in addition to the major component, Al.
Akdalaite has been also identified as a component of bauxite from Weipa, Cook Shire, Queensland, Australia (Tilley and Eggleton, 1996), and as a product of the eruption of the Bulla mud volcano, Bulla Island, Baku Archipelago, near the city of Baku, Azerbaijan (Novgorodova and Mamedov, 1996).
Ferrihydrite, FeO7(OH) = FeFe3+Fe3+O7(OH), is widespread in the soils and different weathered rocks. The cotype localities of ferrihydrite are Leninogorskoe (formerly Ridder) and Belousovskoe Pb–Zn deposits, both situated in northeastern Kazakhstan (Chukhrov et al., 1973). Natural material is insufficiently characterized because it often forms fine mixtures with other minerals and does not form perfect crystals suitable for single-crystal X-ray structure analysis. Ferrihydrite is a nanosized mineral with a long history of debate on its crystal structure, composition, and formation mechanisms (Boily and Song, 2020, and references therein). The first structure models have been obtained for the synthetic ferrihydrite analogue. Manceau and Drits (1993) and Drits et al. (1993) (see also references therein) discussed in detail several suggested models of ferrihydrite (Towe and Bradley, 1967; Harrison et al., 1967; Eggleton and Fitzpatrick, 1988) and supposed that the studied ferrihydrite samples are mixtures of a defect-free phase with the space group P-31c and unit-cell parameters a=2.96 and c=9.40 Å, defective ferrihydrite with the hexagonal supercell with a=5.126 Å, and ultradispersed hematite with the mean dimension of coherent scattering domains of 10–20 Å. The crystal structure of the synthetic ferrihydrite analogue has been refined by modeling of the pair distribution function derived from direct Fourier transformation of the X-ray scattering data (Michel et al., 2007, 2010). The model is consistent with the nolanite-type structure with 20 % tetrahedrally and 80 % octahedrally coordinated iron, space group P63mc. It was also shown that short- and intermediate-range ordering of the naturally occurring ferrihydrite – characterized by high-energy X-ray scattering, pair distribution function analysis, and transmission electron microscopy – is comparable to the synthetic ferrihydrite analogue (Cismasu et al., 2011). Manceau et al. (2014) proposed for ferrihydrite a modified akdalaite structure model in which Fe has only octahedral coordination. Later it was shown that the single-phase model of Michel et al. (2007) matches the experimental data without the need for multi-phase structures (Chappell et al., 2017, and references therein; Sassi and Rosso, 2019). Funnell et al. (2020) evaluated the two principal contending models (a multi-phase system without tetrahedrally coordinated iron and the single-phase model with tetrahedrally coordinated iron) and stated that the multi-phase model requires unphysical structural rearrangements to fit the data, whereas the single-phase one accounts for the data straightforwardly. Thus, the latter provides the more accurate description of the short- and intermediate-range order of ferrihydrite (Funnell et al., 2020).
Ferrihydrite forms an incomplete solid-solution series with akdalaite. With increasing Al and Si contents, a decrease in particle size and an increase in structural disorder were observed (Cismasu et al., 2011).
Minerals belonging to the kamiokite group have the generalized crystal-chemical formula M12O8. Currently, only members of this group with Mo4+ at the M1 site are known.
Kamiokite, FeMoO8 = MoFe2+Fe2+O7O, has been described as a new mineral from the Kamioka mine, Japan (Sasaki et al., 1985). The mineral occurs in quartz-molybdenite stockwork veins associated with granite porphyry dikes. The empirical formula of the holotype sample is FeMnMoO8. According to the results of the crystal structure refinement of a sample from the type locality (Kanazawa and Sasaki, 1986), all Mo4+ in kamiokite occurs at the M1 site. Half of the Fe atoms are in tetrahedral coordination, at the T site, while the other half are in octahedral coordination, at the M2 site.
Another occurrence of kamiokite is the Mohawk mine, Michigan, USA, where this mineral occurs in fissure veins filled during low-grade regional metamorphism of basalt. The empirical formula of kamiokite from the Mohawk mine derived based on electron microprobe analyses and structural considerations is (FeCu0.16FeSi0.08Al0.04Zn0.03)Σ2.00(Mo2.52Fe V0.14FeTi0.07Al0.04)Σ3.00O8 (Johan and Picot, 1986). This formula is charge-balanced under the assumption that vanadium is tetravalent. Taking into account the scheme for assigning ionic species based on the chemical formula (see Appendix A below), this formula should be rewritten as follows:
In this formula, Si is placed at the T site with tetrahedral coordination. However, the presence of Si in the nolanite-type structure is questionable, taking into account that the ionic radius of [4]Si4+ (0.259 Å) is much smaller than those of bivalent cations (e.g., 0.619 Å for [4]Fe2+, 0.586 Å [4]Fe2+) (Hawthorne and Gagné, 2024). The presence of [4]Mo6+ or [6]Mo6+ (with the ionic radii of 0.398 and 0.607 Å, respectively) in the nolanite structure is also unlikely either from the point of view of local charge balance or for steric reasons.
Mg-bearing kamiokite was found in magnetite-bearing assemblages of the Allende meteorite (Ma and Beckett, 2016).
Iseite, MnMoO8 = MoMn2+Mn2+O7O, has been discovered in rhodochrosite veins cross-cutting the Fe–Mn ore of the Shobu deposit, Shima Peninsula, Japan (Nishio-Hamane et al., 2013). The mineral forms minute (up to 20 µm across) lamellar crystals. The powder X-ray diffraction pattern of iseite is close to that of kamiokite. The Rietveld refinement of the crystal structure confirmed the presence of two Mn sites, tetrahedrally and octahedrally coordinated. The empirical formula of the studied sample is Mn1.787Fe0.193Mo3.010O8. Contents of other elements such as Ca, Mg, Ti, and W are below the detection limits.
Majindeite, Mg2MoO8 = MoMgMgO7O, occurs as minute crystals (up to 1 µm across) in the Allende meteorite, which is classified as a CV3 carbonaceous chondrite (Ma and Beckett, 2016). The empirical formula of the studied sample is (Mg1.57Fe0.43)Mo3.00O8. Using data from electron backscatter diffraction, it was shown that majindeite has the P63mc symmetry and is isostructural with kamiokite and iseite. Majindeite is a natural analogue of the synthetic compound Mg2Mo3O8 with the nolanite-type structure (Knorr and Mueller, 1995).
The generalized crystal-chemical formula of minerals belonging to the rinmanite group is (M112+)M2O7(OH). Currently, two members of this group are known.
Rinmanite, (FeMg)Sb5+ZnO7(OH), has been discovered in a skarn assemblage of the Garpenberg Norra Zn–Pb mine, Hedemora, Dalarna, Sweden. Its first description and crystal structure data were reported by Holtstam et al. (2001). The empirical formula of the holotype rinmanite sample written taking into account structural data and the Mössbauer spectrum showing Fe3+ as the only valence state of iron (Holtstam et al., 2001) is (Zn1.58Mn0.31Mg0.06)Σ1.95Sb2.03[(FeAl0.15)Mg1.95)]Σ5.98 O14.01(OH)1.99 (Z=1). The H2O content in this sample was not determined. Recalculation of the sum of 10 M cations taking into account structural data results in the charge-balanced formula M1[(FeAl0.15)Mg1.95Sb0.04)]Σ6.00M2Sb(Zn1.59Mn0.31 MgO[(OH)1.75O0.25]Σ2.00 (according to crystal-chemical criteria, excessive Sb was placed at the octahedrally coordinated Fe3+-dominant M1 site). Both empirical formulae lead to the ideal end-member formula (FeMg2)Sb2Zn2O14(OH)2 (Z=1), in accordance with the accepted rules of chemical identification and classification of minerals (Bosi et al., 2019a, b). In this formula, Mg2+ is the main charge-balancing bivalent cation at the M1 site.
In the recently discovered mineral species approved with the name zincorinmanite-(Zn), zinc is the predominant bivalent component at the M1 site. The crystal-chemical formula of the holotype sample of zincorinmanite-(Zn) is M1[(FeAl0.36)(Zn0.63Mg0.33FeMnTi0.12] M2(Sb1.94Ti0.06)TZnO[(OH)1.23O0.77] (Z=1), and the ideal end-member formula is (FeMg)SbZnO7(OH) (Z=2) (IMA proposal 2023-107). Despite the fact that the name rinmanite-(Zn) was recently approved, it has been changed to zincorinmanite-(Zn), in accordance with recommendations of some IMA CNMNC members. This mineral originates from a metasomatic sulfide-free ore occurrence with chalcophile elements (Zn, Cu, Sb, Pd, As) located near the village of Nežilovo, Pelagonian Massif, Republic of North Macedonia (Chukanov et al., 2015).
All historical mineral names have been preserved. Historical names cannot be changed in order to standardize the nomenclature of a group or supergroup, since mixed nomenclature systems are now accepted by the IMA CNMNC (Hatert et al., 2013).
In new minerals of the nolanite supergroup, each combination of dominant octahedrally coordinated M1 and M2 cations and X anion defines the root name. The subordinate criterion is the dominant charge at the tetrahedral T site. A Levinson-type suffix should be applied to indicate the dominant component at the T site. The same principle of the application of the Levinson suffix should be applied to new members of other groups belonging to the nolanite supergroup.
The charge-balanced general crystal-chemical formula of rinmanite-group minerals, (M112+)M2O7(OH), can be written only with two cations at the M1 site. Currently, for charge-balancing cations, prefixes rather than Levinson-type suffixes are used (e.g., in ferricoronadite, ferrihollandite, and magnesiohögbomite). Thus, in the names of rinmanite-group minerals, the charge-balancing M12+ cation defines the prefix.
Taking into account these rules and recommendations, the following names for new rinmanite-group minerals could be proposed.
6.1 Nolanite group
-
The potential new mineral VV3+AlO7(OH) should have the name nolanite-(Al).
-
The potential new mineral FeFe3+AlO7(OH) should have the name ferrihydrite-(Al).
-
The potential new mineral VFe3+AlO7(OH) should have the name “new root name”-(Al).
-
The potential new mineral VFe3+Fe3+O7(OH) should have the name “new root name”-(Fe3+).
6.2 Kamiokite group
-
The potential new mineral MoFe2+ZnO8 should have the name kamiokite-(Zn).
-
The potential new mineral MoFe2+MgO8 should have the name kamiokite-(Mg).
-
The potential new mineral MoMn2+ZnO8 should have the name iseite-(Zn).
-
The potential new mineral MoMgZnO8 should have the name majindeite-(Zn).
-
The potential new mineral TiMgZnO8 should have the name “new root name”-(Zn).
6.3 Rinmanite group
-
The potential new mineral (FeFe2+)Sb5+ZnO7(OH) should have the name ferrorinmanite-(Zn).
-
The potential new mineral (FeMg)Sb5+MgO7(OH) should have the name magnesiorinmanite-(Mg).
-
The potential new mineral (FeMg)Sb5+Mn2+O7(OH) should have the name magnesiorinmanite-(Mn2+).
-
The potential new mineral (Al2Mg)Sb5+MgO7(OH) should have the name “new root name”-(Mg).
-
The potential new mineral (FeMg)NbMgO7(OH) should have the name “new root name”-(Mg).
If a composition with R4+ at M2 were to be found, that would constitute a new group with X= OH. The general formula of a member of this group would be M12O7(OH) or (M112+)M2O7(OH). In a member of this group, the Levinson-type suffix should be applied to indicate the dominant T2+ or T3+ cation.
For potential new analogues of the known members of the nolanite and rinmanite groups with F instead of OH, the prefix “fluor” should be added. For example, the hypothetic mineral (FeZn)Sb5+ZnO7F should be named fluorzincorinmanite-(Zn); the mineral VV3+Fe3+O7F should be named fluornolanite.
The nolanite supergroup is established. It is divided into the nolanite group, the kamiokite group, and the rinmanite group, in accordance with the highest charge of the species-defining cations and the dominant X species (X= O or OH) (Table 1).
The general crystal-chemical formulae of minerals belonging to the nolanite and rinmanite groups are M12O7(OH) and (M112+)M2O7(OH), respectively.
Minerals with the crystal chemical formulae (M112+)M2O7O and M12O7O are considered members of the kamiokite group.
Potential new nolanite-supergroup minerals with the general formula M12O7(OH) or (M112+)M2O7(OH) would constitute a particular group.
Each combination of the M1 and M2 cations and X anion defines the root name. In the names of new minerals, the Levinson-type suffix should be applied to indicate the dominant component at the T site, whereas the charge-balancing M12+ cation in rinmanite-group minerals defines the prefix.
The rinmanite-group mineral approved recently with the name rinmanite-(Zn) is renamed zincorinmanite-(Zn). All other (historical) names have been preserved.
Relationships between members of each group can be described by homovalent substitution schemes, whereas relationships between different groups are determined only by heterovalent substitution schemes.
Among the species-defining cations in the known nolanite-supergroup minerals, only Fe has a variable valence state. Even in rinmanite and zincorinmanite-(Zn), which crystallized under extremely oxidizing conditions, Mn is bivalent despite the fact that the Fe3+ : (FeFe2+) ratio is close to 1. Therefore, in order to calculate formulae of Fe-bearing members of this group in the absence of structural data, the Fe2+ : Fe3+ ratio should be determined either by direct methods (e.g., using wet chemical analysis, Mössbauer spectroscopy, from the ratio of the intensities of the FeKβ5 and FeKβ1 lines in the X-ray spectrum) or calculated from the charge balance in the empirical formula. In the latter case, direct determination of H2O is very desirable.
The recommended guidelines of the formula calculation are as follows.
- 1.
First, tetrahedral T cations are considered. Their charges are +2 or +3.
- 1.1.
In nolanite-group minerals (i.e., minerals with ), X= OH, and all species-defining cations (including T) are trivalent. Among them, Al shows the most pronounced affinity to the tetrahedral coordination. The occurrence of Fe3+ in tetrahedral coordination is also common. For other possible trivalent cations (e.g., Mn3+, Cr3+, V3+), the tetrahedral coordination is unusual. Therefore, if in a nolanite-group mineral the Al content is >1 atom per formula unit (apfu), one Al cation is placed in T, and the Al is placed in the octahedral sites. If Al <1 apfu, all Al is placed in T. Thereafter, T is completed first by Fe3+ and then (if the content of Fe3+ is insufficient) by admixed bivalent cations in the sequence Zn, Mg, Fe, and Mn (see item 1.2 below). The larger M1 octahedron is then filled with the remaining cations in decreasing order of their ionic radii in octahedral coordination [r(Mn3+) >r(Fe3+) >r(V3+) >r(Cr3+) >r(Al3+)] (Hawthorne and Gagné, 2024), and the rest of the cations are placed in M2.
- 1.2.
In minerals belonging to the kamiokite and rinmanite groups (i.e., minerals with and +5, respectively), dominant T cations are bivalent. As the example of rinmanite shows, Zn has a stronger affinity for tetrahedral coordination than Mg: in this mineral all zinc is at the T site, while most magnesium occurs at the M1 site. Therefore, the filling of T must occur in the following order. First, Zn2+, whose radius in tetrahedral coordination is 0.586 Å (Hawthorne and Gagné, 2024), is placed in T. If there is not enough zinc, then this site is filled with divalent cations with radii closest to the radius of [4]Zn2+ in the order Mg2+ (r=0.573 Å), Fe2+ (r=0.619 Å), and Mn2+ (r=0.680 Å).
- 1.1.
- 2.
In the second step, octahedral sites of minerals belonging to the kamiokite and rinmanite groups are considered.
- 2.1.
If and X= O, the nolanite-supergroup mineral is assigned to the kamiokite group. In this case, the Mo4+ cation with r=0.637 Å is placed in M1, and then M1 is completed with other tetravalent cations (in the order Ti4+ (r=0.605 Å), Sn4+ (r=0.699 Å), Zr4+ (r=0.702 Å)) and then trivalent cations whose ionic radii are the closest to the ionic radius of the dominant tetravalent cation. The rest of the cations are placed in M2.
- 2.2.
If and X= OH, a nolanite-supergroup mineral is assigned to the rinmanite group. In this case, the Sb5+ cation with r=0.611 Å is placed in M2, and then M2 is completed with other pentavalent cations (in the order Nb5+ (r=0.627 Å), Ta5+ (r=0.622 Å), Bi5+ (r=0.744 Å)) and then tetravalent cations whose ionic radii are the closest to the ionic radius of the dominant pentavalent cation. The rest of the cations are placed in M1.
- 2.1.
Synthetic compounds with the nolanite-type structure are characterized by a wide variety of cations (Ansell and Katz, 1966; Yamaguchi et al., 1969; Torardi and McCarley, 1985; Cheetham et al., 1989; Knorr and Mueller, 1995; Michel et al., 2007, 2010; Abe et al., 2010; Zhang et al., 2011; Playford et al., 2013; Morey et al., 2019; Parise et al., 2019; Cook et al., 2020; Prodan et al., 2022; Hao et al., 2023, and references therein).
Heteropolyhedral nolanite-like layers formed by octahedra and tetrahedra were reported for the crystal structures of swedenborgite, NaBe4SbO7 (Huminicki and Hawthorne, 2001, and references therein), and related synthetic compounds. It is noteworthy that these compounds are characterized by the same space group P63mc as nolanite-supergroup minerals and close values of unit-cell parameters. Unlike nolanite-supergroup minerals, in swedenborgite with a=5.4317(2) and c=8.8571(4) Å, heteropolyhedral layers consisting of SbO6 octahedra and BeO4 tetrahedra, sharing common vertices, alternate with the layers of BeO4 tetrahedra. Na+ cations occupy additional sites between the layers (Huminicki and Hawthorne, 2001). Thus, we consider swedenborgite as a mineral related to the nolanite supergroup.
Nolanite-type modules, TM4O7(OH), alternating with spinel-type modules, T2M4O8, where T and M represent tetrahedrally and octahedrally coordinated cations, respectively, occur in the structures of högbomite-supergroup minerals (Hejny and Armbruster, 2002; Armbruster, 2002; Chukanov et al., 2018; Cámara et al., 2018). These minerals belong to the polysomatic series formed by different stacking sequences of the nolanite-type and spinel-type modules. In högbomite-supergroup minerals, charge-balancing cations in the end-member formulae are considered species-defining components. A similar approach has been applied to the rinmanite group within the nolanite supergroup.
No data sets were used in this article.
NVC developed the mineralogical nomenclature of minerals of the nolanite supergroup and carried out literature search. VMG wrote the parts of the article related to the crystal chemistry of the rinmanite group. RKR wrote the parts of the article related to the crystal chemistry of the nolanite group. NVZ developed a general crystal-chemical systematics of minerals of the nolanite supergroup. IVP performed a description of all known finds of minerals of the nolanite supergroup.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “New minerals: EJM support”. It is not associated with a conference.
We thank Ulf Hålenius, Anthony Kampf, and Cristian Biagioni for valuable comments.
This paper was edited by Cristian Biagioni and reviewed by Anthony Kampf and Ulf Hålenius.
This work was performed in accordance with the state task of the Russian Federation, registration number 124013100858-3. Publisher's note: the article processing charges for this publication were not paid by a Russian or Belarusian institution.
Abe, H., Sato, A., Tsujii, N., Furubayashi, T., and Shimoda, M.: Structural refinement of T2Mo3O8 (T = Mg, Co, Zn and Mn) and anomalous valence of trinuclear molybdenum clusters in Mn2Mo3O8, J. Solid State Chem., 183, 379–384, 2010.
Ansell, G. B. and Katz, L.: A refinement of the crystal structure of zinc molybdenum (VI) oxide Zn2MoO8, Acta Crystallogr., 21, 482–485, 1966.
Armbruster, T.: Revised nomenclature of högbomite, nigerite, and taaffeite minerals, Eur. J. Mineral., 14, 389–395, https://doi.org/10.1127/0935-1221/2002/0014-0389, 2002.
Boily, J.-F. and Song, X.: Direct identification of reaction sites on ferrihydrite, Communications Chem., 3, 79, https://doi.org/10.1038/s42004-020-0325-y, 2020.
Bosi, F., Biagioni, C., and Oberti, R.: On the chemical identification and classification of minerals, Minerals, 9, 591, https://doi.org/10.3390/min9100591, 2019a.
Bosi, F., Hatert, F., Hålenius, U., Pasero, M., Miyawaki, R., and Mills, S.: On the application of the IMA−CNMNC dominant-valency rule to complex mineral compositions, Mineral. Mag., 83, 627–632. https://doi.org/10.1180/mgm.2019.55, 2019b.
Cámara, F., Cossio, R., Regis, D., Cerantola, V., Ciriotti, M. E., and Compagnoli, R.: Beltrandoite, a new root-name in the högbomite supergroup: the Mg end-member magnesiobeltrandoite-2N3S, Eur. J. Mineral., 30, 545–558, https://doi.org/10.1127/ejm/2017/0029-2692, 2018.
Chappell, H. F., Thom, W., Bowron, D. T., Faria, N., Hasnip, P. J., and Powell, J. J.: Structure of naturally hydrated ferrihydrite revealed through neutron diffraction and first-principles modelling, Phys. Rev. Mater., 1, 036002, https://doi.org/10.1103/PhysRevMaterials.1.036002, 2017.
Cheetham, K., Hibble, S. J., and Wakerley, H. R.: Comparison between Mo-Mo and W-W bonding in oxide clusters: crystal structure of Zn2MoW2O8 determined by TOF powder neutron diffraction, Inorg. Chem., 28, 1203–1204, 1989.
Chukanov, N. V., Jančev, S., and Pekov, I. V.: The association of oxygen-bearing minerals of chalcophile elements in the orogenetic zone related to the “mixed series” complex near Nežilovo, Republic of Macedonia, Macedonian J. Chem. Chem. Eng., 34, 115–124, https://doi.org/10.20450/mjcce.2015.612, 2015.
Chukanov, N. V., Krzhizhanovskaya, M. G., Jančev, S., Pekov, I. V., Varlamov, D. A., Göttlicher, J., Rusakov, V. S., Polekhovsky, Yu. S., Chervonnyi, A. D., and Ermolaeva, V. N.: Zincovelesite-6N6S, Zn3(Fe3+,Mn3+,Al,Ti)8O15(OH), a new högbomite-supergroup mineral from Jacupica mountains, Republic of Macedonia, Mineral. Petrol., 112, 733–742, https://doi.org/10.1007/s00710-018-0555-1, 2018.
Chukhrov, F. V., Zvyagin, B. B., Gorshkov, A. I., Ermilova, L. P., and Balashova, V. V.: Ferrihydrite, Int. Geol. Rev., 16, 1131–1143, 1973.
Cismasu, A. C., Michel, F. M., Tcaciuc, A. P., Tyliszczak, T., and Brown, G. E.: Composition and structural aspects of naturally occurring ferrihydrite, Comptes Rendus Geosci., 343, 210–218, https://doi.org/10.1016/j.crte.2010.11.001, 2011.
Cook, D. S., Lees, M. R., Fisher, J. M., Thompsett, D., and Walton, R. I.: Ga2.52V2⋅48O7⋅33(OH)0.67, a synthetic member of the nolanite/akdalaite-type family of oxyhydroxides containing trivalent vanadium, J. Solid State Chem., 288, 121396, https://doi.org/10.1016/j.jssc.2020.121396, 2020.
Drits, V. A., Sakharov, B. A., Salyn, A. L., and Manceau, A.: Structural model for ferrihydrite, Clay Miner., 28, 185–207, 1993.
Eggleton, R. A. and Fitzpatrick, R. W.: New data and a revised structural model for ferrihydrite, Clays Clay Miner., 36, 111–124, 1988.
Funnell, N. P., Fulford, M. F., Inoué, S., Kletetschka, K., Michel, F. M., and Goodwin, A.L.: Nanocomposite structure of two-line ferrihydrite powder from total scattering, Communications Chem., 3, 22, https://doi.org/10.1038/s42004-020-0269-2, 2020.
Gatehouse, B. M., Gray, I. E., and Nickel, E. H.: The crystal chemistry of nolanite, (V,Fe,Ti,Al)l0014(OH)2 from Kalgoorlie, Western Australia, Am. Mineral., 68, 833–839, 1983.
Hanson, A. W.: The crystal structure of nolanite, Acta Crystallogr., 11, 703–709, 1958.
Hao, C., Li, X., Huang, H., Ge, L., Fu, Z., Lu, Y., Wang, Y., Zhang, S., and Cheng, Z.: Simultaneous activation of different coordination sites in single-phase FeCoMo3O8 for the oxygen evolution reaction, ACS Energy Lett., 8, 4506–4513, 2023.
Harrison, P. M., Fischbach, F. A., Hoy, T. G., and Haggis, G. H.: Ferric oxyhydroxide core of ferritin, Nature, 216, 1188–1190, 1967.
Hatert, F., Mills, S. J., Pasero, M., and Williams, P. A.: CNMNC guidelines for the use of suffixes and prefixes in mineral nomenclature, and for the preservation of historical names, Eur. J. Mineral., 25, 113–115, https://doi.org/10.1127/0935-1221/2013/0025-2267, 2013.
Hawthorne, F. C. and Gagné, O. C.: New ion radii for oxides and oxysalts, fluorides, chlorides and nitrides, Acta Crystallogr., 80, 326–339, https://doi.org/10.1107/S2052520624005080, 2024.
Hejny, C. and Armbruster, T.: Polysomatism in högbomite: The crystal structures of 10T, 12H, 14T, and 24R polysomes, Am. Mineral., 87, 277–292, 2002.
Holtstam, D., Gatedal, K., Söderberg, K., and Norrestam, R.: Rinmanite, Zn2Sb2Mg2Fe4O14(OH)2, a new mineral species with a nolanite-type structure from the Garpenberg Norra mine, Dalarna, Sweden, Canad. Mineral., 39, 1675–1683, 2001.
Huminicki, D. M. C. and Hawthorne, F. C.: Refinement of the crystal structure of swedenborgite, Canad. Mineral., 39, 153–158, 2001.
Hwang, S. L., Shen, P. Y., Chu, H. T., and Yui, T. F.: A new occurrence and new data on akdalaite, a retrograde mineral from UHP whiteschist, Kokchetav massif, northern Kazakhstan, Int. Geol. Rev., 48, 754–764, 2006.
Johan, Z. and Picot, P.: Kamiokite, Fe2Mo3O8, a tetravalent molybdenum oxide: new data and occurrences, Tschermaks Mineral. Petrogr. Mitt., 35, 67–75, 1986.
Kanazawa, Y. and Sasaki, A.: Structure of kamiokite. Acta Crystallog., C42, 9–11, 1986.
Knorr, R., and Mueller, U.: η-Mo4O11 und Mg2Mo3O8: eine neue Synthese und Verfeinerung ihrer Kristallstrukturen, Z. Anorg. Allgemeine Chemie, 621, 541–545, 1995 (in German).
Liu, P., Gu, X., Zhang, W., Hu, H., Chen, X., Wang, X., Song, W., Yu, M., and Cook, N. J.: Jingwenite-(Y) from the Yushui Cu deposit, South China: The first occurrence of a V-HREE-bearing silicate mineral, Am. Mineral., 108, 192–196, 2023.
Ma, C. and Beckett, J. R.: Majindeite, Mg2Mo3O8, a new mineral from the Allende meteorite and a witness to post-crystallization oxidation of a Ca-Al-rich refractory inclusion, Am. Mineral., 101, 1161–1170, 2016.
Manceau, A. and Drits, V. A.: Local Structure of Ferrihydrite and Feroxyhite by Exafs Spectroscopy, Clay Miner., 28, 165–184, 1993.
Manceau, A., Skanthakumar, S., and Soderholm, L.: PDF analysis of ferrihydrite: Critical assessment of the under-constrained akdalaite model, Am. Mineral., 99, 102–108, 2014.
Michel, M. F., Ehm, L., Antao, S. M., Lee, P. L., Chupas, P. J., Liu, G., Strongin, D. R., Schoonen, M. A. A., Phillips, B. L., and Parise, J. B.: The structure of ferrihydrite, a nanocrystalline material, Science, 316, 1726–1729, https://doi.org/10.1126/science.1142525, 2007.
Michel, F. M., Barrón, V., Torrent, J., Morales, M. P., Serna, C. J., and Boily, J.-F., Qingsong, L., Ambrosinig, A., Cismasu, A. C., and Brown Jr., G. E.: Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism, P. Natl. Acad. Sci. USA, 107, 2787–2792, 2010.
Morey, J. R., Scheie, A., Sheckelton, J. P., Brown, C. M., and McQueen, T. M.: Ni2Mo3O8: Complex antiferromagnetic order on a honeycomb lattice, Phys. Rev. Mater., 3, 014410, https://doi.org/10.1103/PhysRevMaterials.3.014410, 2019.
Nishio-Hamane, D., Tomita, N., Minakawa, T., and Inaba, D.: Iseite, Mn2Mo3O8, a new mineral from Ise, Mie Prefecture, Japan, J. Mineral. Petrol. Sci., 108, 37–41, 2013.
Novgorodova, M. I. and Mamedov, Y. G.: Native aluminum from mud volcano at the Bulla island, Caspian sea, Lithology Mineral Resources, 31, 301–310, 1996.
Parise, J. B., Xia, B., Simonson, J. W., Woerner, W. R., Plonka, A. M., Phillips, B. L., and Ehm, L.: Structural chemistry of akdalaite, Al10O14(OH)2, the isostructural aluminum analogue of ferrihydrite, Crystals, 9, 246, https://doi.org/10.3390/cryst9050246, 2019.
Prodan, L., Filippova, I., Zubtsovskii, A. O., Shova, S., Widmann, S., Tsirlin, A. A., Kézsmárki, I., and Tsurkan, V.: Dilution of a polar magnet: Structure and magnetism of Zn-substituted Co2Mo3O8, Phys. Rev., B106, 174421, https://doi.org/10.1103/PhysRevB.106.174421, 2022.
Playford, H. Y., Hannon, A. C., Barney, E. R., and Walton, R. I.: Structures of uncharacterized polymorphs of gallium oxide from total neutron diffraction, Chem. Eur. J., 19, 2803–2813, 2013.
Robinson, S. C., Evans Jr., H. T.,, Schaller, W. T., and Fahey, J. J.: Nolanite, a new iron-vanadium mineral from Beaverlodge, Saskatchewan, Am. Mineral., 42, 619–628, 1957.
Sasaki, A., Yui, S., and Yamaguchi, M.: Kamiokite, Fe2Mo3O8, a new mineral, Mineral. J. (Japan), 12, 393–399, 1985.
Sassi, M. and Rosso, K. M.: Roles of hydration and magnetism on the structure of ferrihydrite from first principles, ACS Earth and Space Chem., 3, 70–78, 2019.
Shpanov, Ye. P., Sidorenko, G. A., and Stolyarova, T. I.: Akdalaite, a new hydrated variety of alumina, Intern. Geol. Rev., 13, 675–680, 1971.
Taylor, C. M. and Radtke, A. S.: New occurrence and data of nolanite, Am. Mineral., 52, 734–743, 1967.
Tilley, D. B. and Eggleton, R. A.: The natural occurrence of eta-alumina (η-Al2O3) in bauxite, Clays Clay Miner., 44, 658–664, 1996.
Torardi, C. C. and McCarley, R. E.: Synthesis, crystal structures, and properties of LiZn2Mo3O8, Zn3Mo3O8, ScZnMo3O8, reduced derivatives containing the Mo3O13 cluster unit, Inorg. Chem., 24, 476–481, 1985.
Towe, K. M. and Bradley, W. F.: Mineralogical constitution of colloidal “hydrous ferric oxides”, J. Colloid Interface Sci., 24, 384–392, 1967.
Voloshin, A. V., Karpov, S. M., Isaenko, S. I., Chernyavsky, A. V., and Sergeeva, N. E.: Raman spectroscopy of vanadium association minerals in massive sulfide deposits of the pyrrhotite gorge (Kola region, Russia) and Vihanti (Finland), Zapiski Rossiiskogo Mineralogicheskogo Obshchestva (Proc. Russ. Mineral. Soc.), 143, 55–60, 2014 (in Russian).
Yamaguchi, G., Yanagida, H., and Ono, S.: The crystal structure of tohdite, Bull. Chem. Soc. Japan, 37, 1555–1557, 1964.
Yamaguchi, G., Okumiya, M., and Ono, S.: Refinement of the structure of tohdite 5Al2O3⋅H2O, Bull. Chem. Soc. Japan, 42, 2247–2249, 1969.
Zhang, X., Yan, W., and Xie, Y.: Synthetic nolanite Fe2.5V1.5V5.6O16 nanocrystals: a new room-temperature magnetic semiconductor with semiconductor-insulator transition, Chem. Commun., 47, 11252–11254, 2011.
- Abstract
- Introduction
- Minerals belonging to the nolanite supergroup
- Nolanite group
- Kamiokite group
- Rinmanite group
- Mineral names
- Conclusions
- Appendix A: Assignment of ionic species to the key sites based on chemical data
- Appendix B: Minerals and synthetic compounds related to the nolanite supergroup
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Review statement
- Financial support
- References
- Abstract
- Introduction
- Minerals belonging to the nolanite supergroup
- Nolanite group
- Kamiokite group
- Rinmanite group
- Mineral names
- Conclusions
- Appendix A: Assignment of ionic species to the key sites based on chemical data
- Appendix B: Minerals and synthetic compounds related to the nolanite supergroup
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Review statement
- Financial support
- References



