the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
H2 mobility and redox control in open vs. closed hydrothermal oceanic systems – evidence from serpentinization experiments
Colin Fauguerolles
Teddy Castelain
Johan Villeneuve
Michel Pichavant
New hydrothermal experiments in rapid-quench pressure vessels have been performed to investigate the effect of redox state on the serpentinization reaction. The experimental hydrogen fugacity () was controlled by monitoring the mobility of H2 in the reacting system (internal vs. external control). This was achieved by using either Au (H2 impermeable) or AgPd (H2 permeable) capsules and Ar pressurizing gas to control . The experiments were performed with either San Carlos olivine powders or Åheim dunite chips. Water / rock mass ratios of 1–2, a total pressure of 50 MPa, and temperatures of 300 and 350 °C were investigated. Experimental durations of 30, 45, or ≈80 d were imposed. Serpentine production is observed in almost all experiments but is favored at 300 °C under external control. The serpentine–magnetite assemblage is observed in Au capsules (high ) at 300 °C, while the serpentine–hematite(–magnetite) is observed in AgPd capsules (low ). At 350 °C, less H2 is produced than at 300 °C and the serpentine–hematite(–magnetite) assemblage is present in both Au and AgPd capsules. Brucite is absent and this is interpreted to reflect both the initially oxidizing conditions and relatively low serpentine production in our experiments. Differences in product phase assemblages found in this study imply that natural serpentinization reaction mechanisms vary with redox conditions, and consequences for H2 production fluxes and rates can be expected. The high- (reduced) internally controlled experiments simulate low-permeability “closed” oceanic hydrothermal systems. The low- (oxidized) externally controlled experiments are analogous to “open” oceanic hydrothermal systems where serpentinization is driven by tectonically aided infiltration of an external fluid.
- Article
(4627 KB) - Full-text XML
- BibTeX
- EndNote
At slow-spreading ridges, tectonic expansion coupled with low magma production allows the outcropping of large volumes of mantle rocks on the ocean floor (Karson et al., 1987; Cannat, 1993). Interaction between seawater and peridotite leads to important modifications of the physical (i.e., rheology, magnetic signature, porosity, density, and permeability; Toft et al., 1990; Escartín et al., 1997, 2001; Oufi et al., 2002; Iyer et al., 2008; Plümper et al., 2012; Kelemen and Hirth, 2012; Rouméjon and Cannat, 2014; Maffione et al., 2014; Evans et al., 2020; Malvoisin et al., 2020) and chemical (i.e., mineral phase assemblages, compositions of solid and fluid phases; Mével, 2003; Bach et al., 2006; Andreani et al., 2007; Evans, 2008; Cannat et al., 2010; Charlou et al., 2010; Seyfried et al., 2011, 2015; Frost et al., 2013; Klein et al., 2014, 2019; Huang et al., 2017a) properties of mantle rocks at crustal scale – a process named serpentinization.
One of the most distinctive features of serpentinization environments is the generation of large amount of H2 caused by the oxidation of ferrous iron (Fe2+), contained in peridotitic minerals, coupled with the reduction of water (Neal and Stanger, 1983; Abrajano et al., 1990; Charlou et al., 2002). The produced ferric iron (Fe3+) is incorporated into precipitating phases such as serpentine, wherein Fe is either partly or mostly Fe3+ (Klein et al., 2009; Marcaillou et al., 2011; Andreani et al., 2013), as well as in magnetite (), a common phase of serpentinites (Toft et al., 1990; Oufi et al., 2002; Klein et al., 2014; Maffione et al., 2014). In comparison, hematite () has been infrequently observed in CO2-free experimental serpentinizing systems (Godard et al., 2013), although its formation as a reaction product of serpentinization has been theoretically considered (Frost, 1985; Evans et al., 2013; Malvoisin, 2015; Ely et al., 2023). The rare natural occurrences of hematite-bearing serpentinites have been attributed to the transformation of magnetite by fluid rock interactions subsequent to serpentinization (Bach et al., 2004). Awaruite (), another Fe-bearing phase commonly present in serpentinites (Chamberlain et al., 1965; Frost, 1985; Klein and Bach, 2009), implies a reduction of Fe2+. This diversity of solid phases involving Fe0, Fe2+, and Fe3+ highlights the potential of serpentinization process to involve apparently opposed and complex changes in redox state. Yet, apart from a few studies (Frost, 1985; Evans et al., 2013; Frost et al., 2013; Lazar, 2020), changes in redox state in serpentinites have remained largely descriptive (based on mineral ratios) and not interpreted in a quantitative framework involving the redox variables: (oxygen fugacity) and (hydrogen fugacity).
The chemical aspects of serpentinization, i.e., fluid–rock reactions, have been investigated theoretically (Frost, 1985; Evans, 2008; Evans et al., 2013). They have led to numerous experimental (e.g., Martin and Fyfe, 1970; Moody, 1976; Seyfried and Dibble, 1980; Wegner and Ernst, 1983; Janecky and Seyfried, 1986; Berndt et al., 1996; McCollom and Seewald, 2001; Allen and Seyfried, 2003; Seyfried et al., 2007; Marcaillou et al., 2011; Hövelmann et al., 2011; Okamoto et al., 2011; Lafay et al., 2012, 2018; Malvoisin et al., 2012b, a; Mayhew et al., 2013; Klein and McCollom, 2013; Ogasawara et al., 2013; Malvoisin and Brunet, 2014; Klein et al., 2015; McCollom et al., 2016, 2020a, b; Syverson et al., 2017; Lamadrid et al., 2017, 2021; Huang et al., 2017a, b, 2019; Tutolo et al., 2018; Oyanagi et al., 2020; Klein and Le Roux, 2020) and thermodynamic studies, the latter based on reaction path models (Sleep et al., 2004; McCollom and Bach, 2009; Klein et al., 2009, 2013; Malvoisin, 2015; Ely et al., 2023). The importance of reaction kinetics has been recently demonstrated (Seyfried et al., 2007; Malvoisin et al., 2012a; Godard et al., 2013; Lamadrid et al., 2017, 2021; Tutolo et al., 2018; Oyanagi et al., 2020), and the evolution of the Fe speciation has been monitored during fluid circulation and fluid–rock interaction (Marcaillou et al., 2011; Andreani et al., 2013; Syverson et al., 2017). However, serpentinization is a reactive transport process which combines rock deformation, fluid circulation, and fluid–rock reaction (Steefel et al., 2005; Andreani et al., 2007; Godard et al., 2013; Klein et al., 2015). Natural serpentinizing systems are open to external fluids inputs. Temperature, water rock ratios (), and redox variables can vary with time and space, leading to variable extents of serpentinization and H2 fluxes (Andreani et al., 2013). Thus, there is an increasing need to couple the chemical and the physical aspects of serpentinization, in particular the hydrodynamic regime of fluid circulations.
The impact of the fluid rock ratio on serpentinization has been explored in several thermodynamic studies (Klein et al., 2013; Malvoisin, 2015; Ely et al., 2023). Comparatively few efforts have been put into experimentally coupling fluid–rock reaction and fluid circulation (Godard et al., 2013; Farough et al., 2016; Tutolo et al., 2018; Peuble et al., 2019; Osselin et al., 2022). In this paper, we present a set of hydrothermal experiments that enable the effect of H2 mobility on the serpentinization reaction to be discussed. Conceptually, the mobility of H2 is used as a proxy of fluid circulation, open vs. closed, in oceanic hydrothermal systems. H2 is the closest to a perfectly mobile component (i.e., components whose chemical potential in the system is imposed by an external reservoir; Weill and Fyfe, 1964; Korzhinskii, 1965), and is the key redox variable in hydrous systems (e.g., Eugster, 1957; Scaillet et al., 1992). In this paper, experiments have been designed in an original way enabling the mobility of H2 to be controlled and varied. Below, the experimental and analytical methodologies are presented and the experimental result detailed. We show that significant differences appear in experimental phase assemblages and proportions, and in extents of reaction depending on H2 mobility, and discuss the implication for serpentinization processes at mid-ocean ridges (MORs).
In the following, redox states will be expressed preferentially in terms of and to a lesser extent in terms of . The is a directly measurable variable (Shaw, 1963), and redox states can be conveniently varied experimentally by changing the (Chou, 1987; Chou and Cygan, 1990; Scaillet et al., 1992; Schmidt et al., 1995). In hydrous systems, the two redox variables, and , are related through the following equilibrium:
whose equilibrium constant (K(R1)) is
where is the fugacity of water. From Eq. (1), can be expressed as
which illustrates that, for a hydrothermal fluid at constant T, , and total pressure (PTot), varies linearly opposite to . The mole fractions of the fluid components (H2, O2, and H2O) are related to their respective fugacities by the following general relation:
where ϕi is the fugacity coefficient of component i and Xi the mole fraction of i in the fluid mixture. For most common geological fluids, is negligible, whereas is readily measurable, reaching values in the same range as under very reducing conditions (Eugster and Skippen, 1967; Frost et al., 2013). For example, at 300 °C and 50 MPa, MPa for an oxidizing corresponding to the hematite–magnetite (HM) buffer, while, for a reducing corresponding to the fayalite–magnetite–quartz (FMQ) buffer, MPa (Table 1).
Table 1Calculated and for HM and FMQ buffers at 300 and 350 °C and 50 MPa.

Calculations are made using a Lewis and Randall mixing rule for the H2O–H2 fluid. a From Shaw and Wones (1964). b From Burnham et al. (1969). c Calculated with SUPCRT92 from Johnson et al. (1992). d From Myers and Eugster (1983) in Chou (1987). e From Haas and Robie (1973) in Chou (1987).
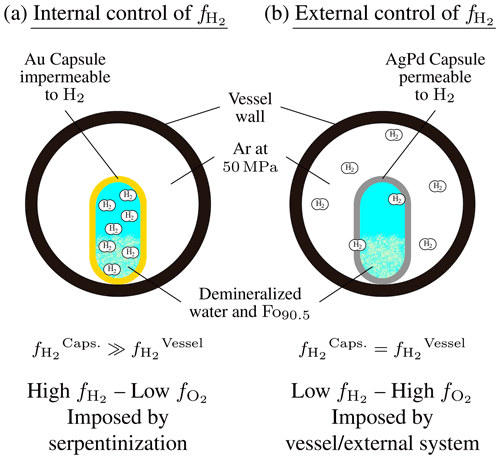
Figure 1Illustration of the two types of experimental control developed in this study. (a) Internal control of . The serpentinization reaction takes place inside an Au capsule impermeable to H2. This allows accumulation of H2 inside the capsule along with advancement of the reaction and leads to high and low . (b) External control of . The serpentinization reaction is performed in an AgPd capsule permeable to H2. The external fluid (Ar pressurizing gas) imposes a constantly low to the system. H2 generated inside the capsule by the reaction is continuously extracted. This leads to low and high .
Below, two cases will be considered depending on how the variable is controlled. In the first case, the variable is controlled internally; i.e., H2 is produced by the advancement of the serpentinization reaction and allowed to accumulate in the reacting system (internal control; Fig. 1a). In the second case, is imposed externally from outside the reacting system (external control; Fig. 1b).
3.1 Starting materials
Two distinct starting materials were used in the experiments: San Carlos olivine and Åheim dunite. The San Carlos olivines (Fo90.5; see Table A1 in Appendix A for detailed composition) were separated by hand from slightly disaggregated San Carlos xenoliths. In order to eliminate all contaminants (spinels, pyroxenes), only grains with a sufficient size (larger than ≈500 µm to allow a precise examination with binoculars) were kept. After several ultrasonic cleanings followed by abundant rinsing with deionized water, all crystals suspected of surface or inclusion contamination were removed. The Åheim dunite contains, in addition to olivine (Fo94.0; Table A1), about 10 vol % orthopyroxene (En93.9; Table A1), 0.5 vol %–1 vol % chromite, and 1 vol %–5 vol % clinochlore. Hydrous silicates such as serpentine, talc, phlogopite, and tremolite have been reported to be present in trace amounts (Berckhemer et al., 1982; Jackson et al., 1992), although none were found from SEM examination. In the same way and in contrast with the observations of (Jackson et al., 1992), no magnetite was observed in our Åheim dunite sample. This is consistent with the magnetic data (low magnetite mass fraction (MMF) of wt %; see Sect. 3.4).
3.2 Experimental charges and capsules
Experimental parameters are summarized in Table 2. The San Carlos olivines were used in powder form, obtained by grinding the olivine crystals in an agate mortar. The resulting powders were sieved to different granulometric fractions: 0.5–1 mm, 45–50 µm, 25–32 µm, and <10 µm. Above 32 µm, granulometric separations were performed with stainless-steel sieves. Below 32 µm, granulometric separations were performed with nylon sieves and a vibrating table. In both cases, powders were abundantly rinsed with deionized water in order to remove the smallest particle sizes. The Åheim dunite was used as experimental starting materials in the form of millimeter-sized chips. Experimental charges, consisting of either the olivine or the dunite (R) plus demineralized water (W), were loaded in noble metal capsules (4.2 or 5 mm diameter, 30–35 mm length, wall thickness 0.2 mm) which were welded shut. Capsules were weighted at each step during preparation and cooled during welding to ensure that no water loss occurred. Masses of reactants were adjusted to yield ratios of either 1 or 2 (Table 2). Capsules were checked for leaks (by placing them in an oven and weighting) and then inserted in the pressure vessel.
Table 2Experimental parameters and summary of results obtained on solid products.
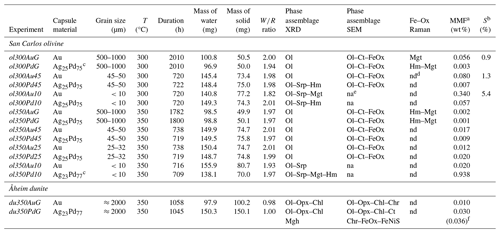
a MMF: magnetite mass fraction, calculated as explained in text. b S: reaction progress, calculated with model proposed by Malvoisin et al. (2012b); see text. c Ag23Pd77 and Ag25Pd75 capsules are indifferently used (the differences of composition are too small to lead a perceptible effect in terms of H2 permeability). d nd: not determined. In spite of numerous attempts the type of iron oxides of these samples could not be determined; see text. e na: not analyzed. f Value in brackets corresponds to the mass fraction of maghemite (considering the maghemite as the only one responsible for the increase of Js; see text). Mineral abbreviations: Ol: olivine; Opx: orthopyroxene; Chl: clinochlore; Srp: serpentine; Ct: chrysotile; FeOx: unspecified Fe oxide; Mgt: magnetite; Hm: hematite; Mgh: maghemite; Chr: chromite; FeNiS: Fe–Ni sulfide.
Table 3 and corresponding and ΔHM calculated using previously published associated with Fo88–91 serpentinization.

a Calculated with SUPCRT92 from Johnson et al. (1992). b Values in bold are calculated by linear interpolation of at 720 h, the duration of the majority of our experiments. c See text for details about the range in Au capsules.
Control of was achieved by using two different capsule materials, Au and Ag25Pd75 or Ag23Pd77 (wt %; AgPd in the following text). Au capsules are almost impermeable to H2 at the temperatures investigated in this study (Gunter et al., 1987). Therefore, in experiments performed with Au capsules, the is intrinsically controlled by and depends on the advancement of the serpentinization reaction (internal control). H2 accumulates inside the capsule, and this leads to high- and thus low- conditions (Fig. 1a). Our experimental setup does not allow a direct measurement of inside Au capsules. However, the can be estimated from the H2 molality in hydrothermal fluids () determined in other closed-system olivine serpentinization experiments (Berndt et al., 1996; Allen and Seyfried, 2003; McCollom et al., 2016; Fauguerolles, 2016). The and the are related by
where K(R2), computed in the following using SUPCRT92 (Johnson et al., 1992), is the equilibrium constant of
and is the molar activity coefficient of H2(aq). The latter can be assimilated to the molar activity coefficient of CO2(aq) (Garrels and Thompson, 1962), itself calculated from the work of Drummond (1981). Experimental data used to constrain in our closed-system experiments are detailed in Table 3. When interpolated at 720 h, the typical duration of our experiments, at 300 °C varies between 4.78 and 0.18 MPa, which corresponds to and , respectively. Differences in between studies reflects the difference in grain size, smaller in the experiments of Berndt et al. (1996) and Fauguerolles (2016), which increases the reaction rate and associated H2 production and increases . Reaction path models (Klein et al., 2013) predict a maximum production of H2 around 322 °C followed by a drastic decrease at higher temperatures. This significant drop in is observed between 300 and 320 °C by McCollom et al. (2016), from MPa () at 300 °C to MPa () at 320 °C, and by Fauguerolles (2016) between 300 and 350 °C, from to 0.23 MPa ( to −5.63). We also note that the 400 °C experiment of Allen and Seyfried (2003) yields the same (0.032 MPa) as that of McCollom et al. (2016) at 320 °C. Based on these results, the in our closed-system experiments can be estimated to be bracketed between 0.18 and 4.78 MPa at 300 °C and between 0.032 and 0.23 MPa at 350 °C.
In contrast, AgPd capsules are quite permeable to H2. At 300 °C, H2 permeability is 9 log units higher in AgPd than in Au and 8 log units higher at 350 °C (Gunter et al., 1987). By using a H2 permeable capsule, the inside the capsule becomes rapidly equal to the outside the capsule (i.e., of the pressurizing gas). Volume considerations (the volume of pressurized gas in the vessel is about 10 cm3, whereas the volume of the experimental capsule is about 0.1 cm3) dictate that, in this case, the within the capsule is imposed by the of the pressurizing gas (external control; Fig. 1b). The imposed by the Ar pressurizing gas has been constrained both directly and indirectly (see Appendix B). At 350 °C, 50 MPa, the is MPa, which corresponds to . At 300 °C, no such measurements are available, but the temperature dependence of the in such an oxidizing range is small, and a maximum of MPa (corresponding to the value of the HM buffer at 300 °C; Table 1) can be assumed. Therefore, our internally and externally controlled experiments differ by 3.45–4.4 log units in (5.9–8.8 log units in ) at 300 °C and, at 350 °C, by 2.55–3.4 log units in (5.1–6.8 log units in ).
Below, experiments will be designated by type of starting material (ol for San Carlos olivine, du for Åheim dunite), temperature (300 for 300 °C, 350 for 350 °C), capsule material (Au for Au, Pd for AgPd), and initial grain size (G for 0.5–1 mm, 45 for 45–50 µm, 25 for 25–32 µm, 10 for <10 µm). For example, ol300Pd45 is the experiment conducted with San Carlos olivine at 300 °C in an AgPd capsule and for an initial grain size of 45–50 µm (Table 2).
3.3 Experimental equipment
In this study, all experiments were performed in René 41 rapid-quench cold-seal pressure vessels (Rudert et al., 1976; Pichavant, 1987; Schmidt et al., 1995) pressurized with Ar. Experimental temperatures, monitored permanently with an external thermocouple, were controlled with a Eurotherm regulator. Temperatures inside the vessel were calibrated under pressure using two thermocouples (precise to ±2 °C according to the manufacturer), spaced 3 cm apart and positioned at the points occupied by the ends of the capsule. In order to minimize the thermal gradient within the capsule, the vessel was positioned in the furnace so that the temperature difference between the calibration thermocouples was less than 1 °C. Pressure was read with a tube manometer to within ±2 MPa. In this study, pressure was kept constant (50 MPa) and two temperatures, 300 and 350 °C, were investigated. Experiments lasted for 30, 45, or 80 d (Table 2). Once completed, the experiment was quenched by removing the pressure vessel from the furnace and allowing it to cool for ≈1 h down to room conditions. When cold, the vessel was opened and the capsule extracted. It was cut parallel to its long axis so that the entire sample volume was exposed and recovered.
3.4 Analytical methods
Compositions of the starting San Carlos olivine and the starting olivine and orthopyroxene of the Åheim dunite have been determined with a Cameca SX Five electron microprobe (ISTO, Orléans). Analyses were carried out in punctual mode using a 15 kV acceleration voltage, a 6 nA sample current, and a 10 and 5 s acquisition time on peak and background respectively. Solid products recovered after the experiments were dried at 110 °C for 24 h. A fraction was ground and mounted in a capillary for X-ray diffraction (XRD) analysis. An Inel CPS120 diffractometer equipped with a curved detector and a Co anticathode was used (ISTO, Orléans). Magnetic data including hysteresis parameters (saturation magnetization: Js; saturation remanent magnetization: Jrs; intrinsic coercivity: Hc; remanent coercivity: Hcr) were obtained using a vibrating sample magnetometer at room temperature (MC MicroMag 3900 Series, IPGP Paris). The magnetite mass fraction (MMF) was obtained using the calibration between the amount of magnetite and Js (proportionality factor of 92 A m2 kg−1 determined from powder mixtures made of synthetic forsterite and magnetite; Malvoisin et al., 2012b). Progress of the serpentinization reaction was calculated from the amount of magnetite produced (MMF) using model 3 of (Malvoisin et al., 2012b). Another fraction of experimental products was selected for detailed textural characterization and phase identification using scanning electron microscopy (SEM) and Raman micro-spectroscopy. Run products were either embedded in epoxy and polished or directly glued on a glass plate. The SEM of the joint BRGM-ISTO facility (Tescan Mira 3 XMU, ISTO Orléans) was used both in secondary (SE) and back-scattered electron (BSE) modes. To reduce charging effects, samples were sputtered with carbon before analysis. Raman characterization of oxides was performed using a Jobin-Yvon Labram HR800 Raman spectrometer (LPG, Nantes) equipped with a CCD detector, a 532 nm solid-state laser, a 1800 grooves mm−1 grating, and a 100× Olympus objective in confocal mode. Acquisition times were 5×60 s. To avoid oxide deterioration (de Faria et al., 1997), the laser beam power was optimized on magnetite and hematite powders provided by Sigma Aldrich and comparable in size to those observed in our synthesized products. Thus, a laser power output set at 50 mW (corresponding to 1.5 mW at sample level) was used in conjunction with a filter reducing the laser power by a factor 10. Raman spectra of iron oxides could be acquired only on samples with the largest grain sizes, after pre-localization of iron oxides using SEM images.
Iron oxide characterization (nature and proportion) was carried out by coupling four methods: SEM, XRD, Raman micro-spectroscopy, and magnetic properties. SEM informed on the presence of iron oxides and on textural relations with other phases and provided a relative quantification of their abundances in the samples. Identification of hematite was based on XRD and Raman data. The distinction between magnetite and maghemite (), while being ambiguous from XRD (Malvoisin et al., 2012b), was achieved from the Raman data, whereas the magnetic properties, in particular the Js measurements, allowed quantification of amounts of magnetite or maghemite present.
Due to the low serpentine abundances and the similarities between XRD spectra of chrysotile and lizardite, it was not possible to clearly discriminate between the different serpentine polymorphs by this method. In contrast to iron oxides, attempts to characterize serpentine polymorphs by Raman spectroscopy (Lemaire, 2000; Auzende et al., 2004; Schwartz et al., 2013; Rouméjon et al., 2015) were inconclusive, and the identification of these polymorphs in this study is based on textural parameters, notably the characteristic acicular habit (fibrous texture) of the chrysotile.
4.1 San Carlos olivine
Fourteen experiments, half in Au and half in AgPd and covering a range of initial grain sizes, durations, and temperatures are reported in Table 2.
4.1.1 Textures
Textures of experimental products are illustrated in Fig. 2. For the coarser initial grain sizes (0.5–1 mm), serpentinization products are concentrated within the starting olivine crystals, in large veins (Fig. 2a and b) or gulfs (Fig. 2c) rather than at its free surface. Vein widths are approximately 5 µm, and two different vein types can be distinguished from SEM observations on orthogonal sections. The first type is mainly composed of fibrous material, interpreted to be chrysotile (see Sect. 3.4). Iron oxides occur as small crystals irregularly dispersed along the vein. The vein is not completely filled by product phases, and there is a void between olivine and the material crystallized in the vein (Fig. 2a). The second type, only observed in Au capsules, is texturally similar to the first, being also symmetrical with respect to the center of the vein. It comprises chrysotile and iron oxides, but the fibrous phase displays a contrast difference: lighter near the olivine and darker in the center of the vein. As for the first type, a void is observed between the light band and olivine (Fig. 2b). Whatever the type of vein, iron oxides are almost always sandwiched between olivine and serpentine (Fig. 2a and b). This textural relationship between minerals is also observed in dissolution gulfs present at the surface of the crystals (Fig. 2c). An extensive search for brucite in the veins with SEM and Raman proved negative.
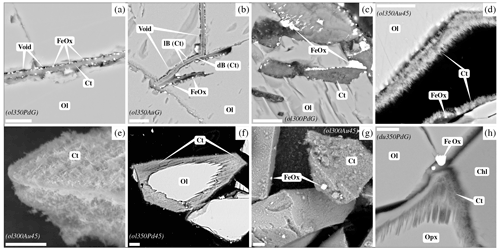
Figure 2SEM images of reacted San Carlos olivines (a–g) and Åheim dunite (h). For each view, the scale bar is 10 µm. Mineral abbreviations are the same as in Table 2. (a–d, f, h) Polished sections and (e, g) unpolished preparations. (a) Vein developed in San Carlos olivine crystal; the middle of the vein is occupied by fibrous chrysotile; iron oxides are concentrated in the void left between olivine and the chrysotile band (BSE, ol350PdG). (b) Texture similar to (a) except that the band is filled with chrysotile showing different contrast (light: lB and dark: dB; BSE, ol350AuG). (c) Dissolution gulf filled with large iron oxides and chrysotile (BSE, ol300PdG). (d) ≈5 µm thick chrysotile layer surrounding olivine crystal and containing <1 µm iron oxides in very limited amounts (BSE, ol350Au45). (e) Fibrous texture of chrysotile (SE, ol300Au45). (f) Chrysotile layer rimming olivine crystal (ol350Pd45). (g) Chrysotile and numerous iron oxides with sizes up to 5 µm developed on the surface of olivine crystals (BSE, ol300Au45). (h) BSE image of dunite reacted in AgPd capsule (du350PdG). Orthopyroxene is more markedly dissolved than olivine. The iron oxide is most probably maghemite (see text). The fibrous material developed at the grain interface is chrysotile.
For smaller initial grain sizes, fibrous chrysotile develops on the surface of the residual olivine crystals where it forms a 5 µm layer (Fig. 2d and e). Olivine can be completely rimmed by serpentine (Fig. 2f). Other reaction products include iron oxides, present locally in very different proportions and sizes (Fig. 2d and g). Brucite is absent.
4.1.2 Mineral assemblages
Olivine, detected by XRD in all charges, persists in all experimental products (Table 2). Similarly, serpentine and iron oxides are present in all experimental charges on the basis of SEM observations (Fig. 2), although they are detected by XRD in only a few experiments (Table 2). Serpentine is detected by XRD in all charges with the smallest initial grain sizes (10 µm) and in only one with an intermediate initial grain size (ol300Pd45; Fig. 3, Table 2). It coexists on XRD patterns with iron oxides in most charges (ol300Pd45, ol300Au10, ol300Pd10 at 300 °C; ol350Pd10 at 350 °C) and is more rarely found in isolation (ol350Au10 at 350 °C; Fig. 3, Table 2). Examination of XRD patterns suggest that serpentine is produced in generally small amounts, except in ol300Pd10 (Fig. 3). Despite extensive tracking with SEM using EDS (energy-dispersive X-ray spectroscopy) and WDS (wavelength-dispersive spectrometry) element maps, brucite was not found and must be considered as absent from San Carlos charges.
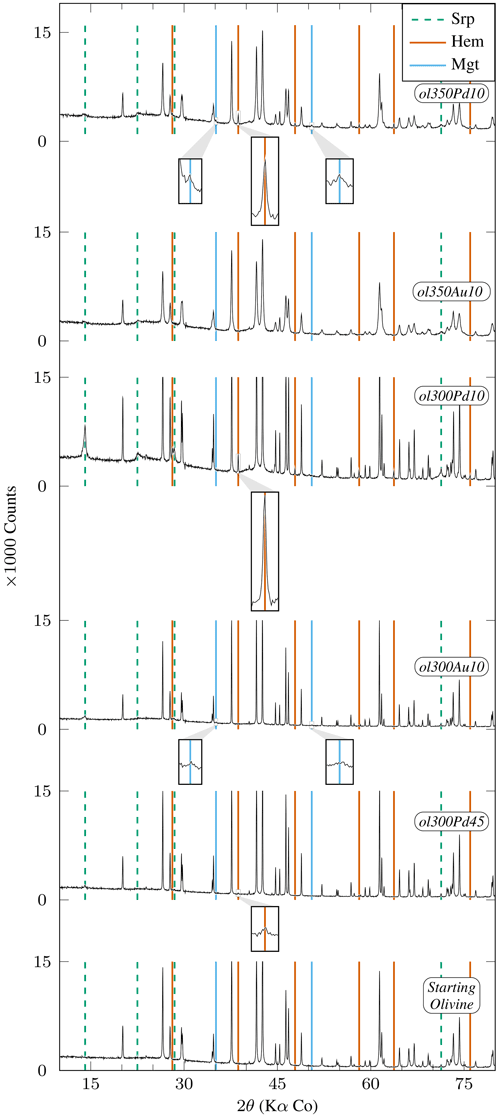
Figure 3XRD diffractograms of experimental charges produced from San Carlos olivine emphasizing phases other than primary olivine (Table 2). Mineral abbreviations are the same as in Table 2. Peaks which are not highlighted correspond to olivine. All peaks higher than 15 000 counts are clipped. Insets are ×6 magnifications of diffractograms. San Carlos olivine is still present at the end of all experiments. Relative olivine and serpentine peak intensities demonstrate limited formation of serpentine except in ol300Pd10. ol300Pd45 is the only charge with initial grain size >10 µm showing detectable serpentine and hematite peaks (inset centered on the 38.71° 2θ peak). Relative peak intensities suggest a more advanced reaction in ol300Pd10 (<10 µm initial grain size) than in ol300Pd45 (45–50 µm). Hematite is identified in experiments performed in AgPd capsules (ol300Pd10, ol350Pd10, and ol300Pd45). Peaks observed in ol350Pd10 and ol300Au10 at 35.13° 2θ and 50.52° 2θ (insets) are attributed to magnetite (see text).
Experimental iron oxide products include magnetite and hematite. Magnetite was positively identified by Raman in the four experiments with coarse initial grain sizes, performed both in Au (ol300AuG, ol350AuG) and AgPd (ol300PdG, ol350PdG; Fig. 4, Table 2). Maghemite (spectrum characterized by a doublet at 665 and 621 cm−1, Legodi and de Waal, 2007, Fig. 4) was not found despite extensive search. In experiments performed with small initial grain sizes in Au at 300 °C (ol300Au10) and in AgPd at 350 °C (ol350Pd10), the iron oxide peaks at 35.13° 2θ and 50.52° 2θ in XRD spectra are attributed to magnetite (Fig. 3, Table 2) because maghemite is absent in the corresponding experiments performed with large initial grain sizes (respectively ol300AuG and ol350PdG, Fig. 4). Following the same reasoning, the increase of Js (Table 4) relative to this of the starting olivine (baselines in Fig. 5) is attributed to the presence of magnetite and allows the calculation of magnetite mass fraction (MMF) in products (Table 2). Magnetic measurements thus highlight the formation of magnetite in two supplementary samples: ol300Au45 and ol300Pd10 (Fig. 5a, Table 2). They are also in good agreement with the XRD measurements since the two samples with the highest MMF are those for which magnetite is detected by XDR (Fig. 3, Table 2). Magnetite is thus confirmed in eight charges, half in Au half in AgPd, while it forms preferentially at 300 rather than 350 °C (five vs. three occurrences, respectively).
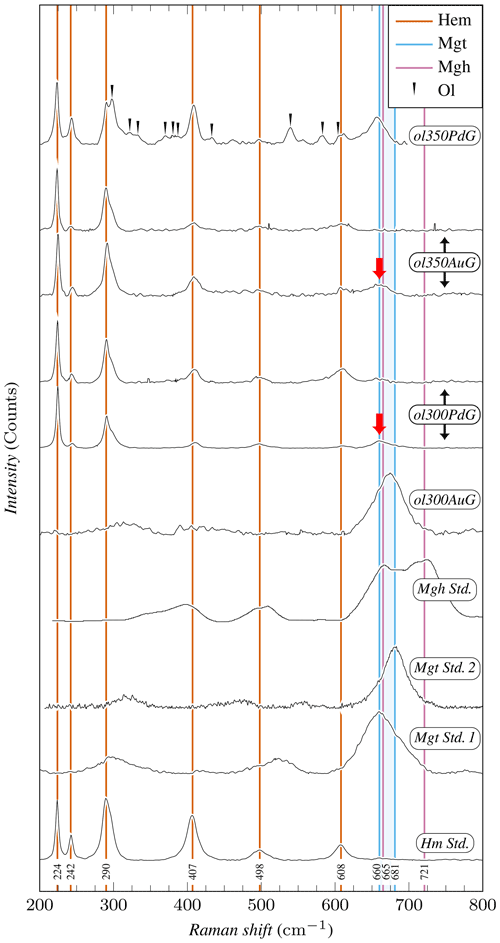
Figure 4Representative Raman spectra of iron oxides. Raman spectra have been normalized to 1 based on the maximum intensity recorded for each spectrum. Mineral abbreviations are the same as in Table 2. Hm Std. and Mgt Std. 1 spectra have been acquired on hematite and magnetite standard powders, respectively. Mgt Std. 2 spectrum corresponds to the R061111 magnetite sample from the RRUFF database (rruff.info, 2023), while maghemite standard (Mgh Std.) comes from Legodi and de Waal (2007). Maghemite is characterized by a doublet at 665 and 621 cm−1, while magnetite is characterized by a single peak in the 660–681 cm−1 range (the area colored in blue delimits the magnetite peak position in Mgt Std.1 and Mgt Std. 2). None of the samples studied contains maghemite, while magnetite is present in ol300AuG and ol350PdG and also in lesser amounts in ol300PdG and ol350AuG (red arrows). Except in ol300AuG, hematite is clearly identified in the three other samples.
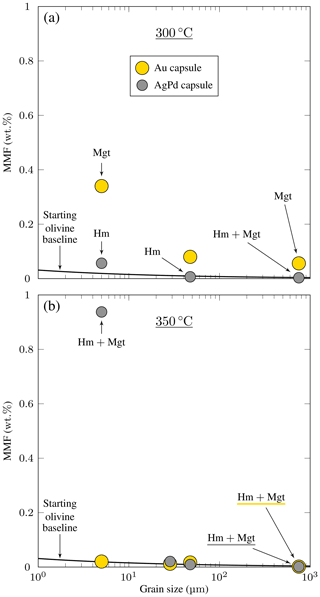
Figure 5Amounts of magnetite in reaction products (magnetite mass fraction, MMF, Table 2) plotted against the initial grain size for the San Carlos olivine experiments. Experiments are distinguished by capsule material, either Au or AgPd. The method for constructing the starting olivine baseline is detailed in Appendix C. Hm and Mgt correspond to hematite and magnetite, respectively. (a) 300 °C charges. Note the systematic increase of MMF with decreasing grain size observed in experiments with Au capsules. Experiments with AgPd capsules show a modest increase of MMF < 10 µm. (b) 350 °C charges. No significant increase of MMF is observed except for the experiment carried out with the smallest initial grain size in AgPd capsule (ol350Pd10).
Table 4Hysteresis measurements.

a Js and Jrs are weighted by the analyzed sample mass. b na: not analyzed.
Hematite was identified by Raman in three of the four coarse-grained samples analyzed (ol300PdG, ol350AuG, ol350PdG; Fig. 4, Table 2) and by XRD in three charges: two with the smallest initial grain sizes (ol300Pd10, ol350Pd10) and, only one, same as for the serpentine, with intermediate initial grain sizes (ol300Pd45; Fig. 3, Table 2). Hematite is preferentially associated with the AgPd experiments. It is absent in Au at 300 °C (ol300AuG) but present in the corresponding charge at 350 °C (ol350AuG; Fig. 4, Table 2). A total of six charges, half at 300 °C and half at 350 °C, contain hematite.
4.1.3 Magnetic properties
The hysteresis data for experimental products (Table 4) are plotted in a vs. diagram (Day diagram; Fig. 6, Day et al., 1977) where is the ratio of saturation remanent magnetization to saturation magnetization and the ratio remanent coercivity to intrinsic coercivity. The Day diagram is a useful way to discriminate between various magnetic mineralogies and grain sizes using magnetization and coercivity parameters (Dunlop, 2002; Malvoisin et al., 2012b). For magnetite, the single-domain (SD, and , Fig. 6) corresponds to particle sizes smaller than ≈ 0.05–0.08 µm, while the multidomain (MD; and > 4, Fig. 6) is reached for magnetite particles larger than 3–15 µm (Oufi et al., 2002). Between these two grain sizes, the magnetic properties evolve in the pseudo-single-domain (PSD) transition zone. The magnetic properties of our experimental samples (only those with MMF above the baselines; see Fig. 5) are compared with those from other experimental studies and natural serpentinites from the literature. All our experimental magnetites plot in the PSD field (Fig. 6). Magnetites produced in Au capsules at 300 °C are in good agreement, in terms of , with other experimental magnetites at 300 °C (Malvoisin et al., 2012b), although the latter extend to higher values (Fig. 6). Magnetites from AgPd have systematically higher than magnetites from Au capsules (Fig. 6). Taken together, the experimental magnetites follow a trend broadly similar to that of magnetites from natural systems (Oufi et al., 2002), although, for a given , the experimental magnetites plot at higher than the natural (Fig. 6).
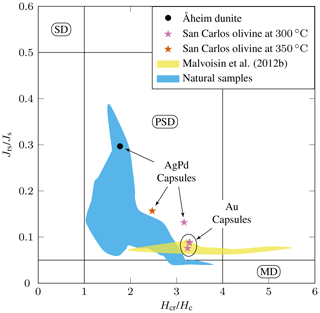
Figure 6 as a function of plot (Day diagram, Day et al., 1977) for selected experimental products. Only charges with magnetite mass fractions (MMFs) higher than the baseline (see Fig. 5) and thus containing a substantial amount of magnetite are plotted. SD: single domain, PSD: pseudo single domain, and MD: multi domain. The yellow field corresponds to the experiments of Malvoisin et al. (2012b) on San Carlos olivine plus pure water at 300 °C. The blue field corresponds to natural serpentinite samples (DSDP Sites 395, 556, 558, and 560 and ODP Sites 670, 895, and 920, as compiled by Oufi et al., 2002).
4.1.4 Influence of capsule material, temperature, and grain size
All 300 °C charges contain serpentine and iron oxides irrespective of the capsule material. However, charge ol300Pd10 has the strongest serpentine XRD signal in our sample set (Fig. 3). In comparison, serpentine is only present in small amounts in the 350 °C charges, whether performed in Au or AgPd (ol350Au10, ol350Pd10, Fig. 3). Therefore, the data suggest a decrease in serpentine production upon increasing temperature from 300 to 350 °C and a higher serpentine production in AgPd than in Au at 300 °C. This latter statement is supported by the XRD detection of serpentine for intermediate grain sizes only in AgPd (ol300Pd45; Fig. 3), although in smaller amounts than for ol300Pd10. We conclude that serpentine preferentially forms at 300 °C in AgPd capsules and in experiments performed with small initial grain sizes.
A first-order change in the nature of the iron oxide phase is observed between the Au and the AgPd experiments at 300 °C. The former contains magnetite and the latter hematite, although a small amount of magnetite is present together with hematite in ol300PdG and ol300Pd10 (Figs. 4, 5, Table 2). This subdivision between Au and AgPd experiments is less strongly marked at 350 °C since hematite coexists with magnetite in ol350AuG, ol350PdG, and ol350Pd10, but magnetite is present in low amounts in ol350AuG (Fig. 4, Table 2). SEM observations show that iron oxides are generally more abundant and grow bigger at 300 °C (up to 5 µm) than at 350 °C (≤1 µm; Fig. 2a–d and g), consistent with the evolution of magnetic properties with increasing temperature. At 350 °C, magnetite has a lower and higher than at 300 °C, which indicates a trend toward the SD field and thus smaller grain sizes (Fig. 6). The magnetite production increases with decreasing grain size at 300 °C in Au (Fig. 5a, Table 2). The same type of evolution is observed in AgPd, although the low MMFs (Fig. 5a) indicate that magnetite production is much more limited than in Au. In comparison, at 350 °C, no such increase of the MMF is observed in Au (Fig. 5b and Table 2) and iron oxides occur in amounts insufficient to be detected by XRD (Table 2). In ol350AuG, the Raman data show that hematite is the predominant iron oxide and that magnetite is present only in small amounts (Fig. 4). MMFs are also low in AgPd at 350 °C (Fig. 5b), indicating a very limited magnetite production at the exception of the charge performed with the smallest grain size (ol350Pd10; Table 2), discussed separately below. We conclude that magnetite preferentially forms at 300 °C in Au rather than in AgPd and in experiments performed with small initial grain sizes.
Hematite is encountered in all 300 and two 350 °C experiments performed in AgPd and in only one in Au at 350 °C (ol350AuG; Figs. 3, 4, Table 2). Relative XRD peak intensities in ol300Pd45 and ol300Pd10 (Fig. 3) imply that hematite production increases with decreasing initial grain size. At 350 °C (ol350Pd45), hematite is not detected by XRD at the difference of at 300 °C (ol300Pd45) for the same initial grain size (Fig. 3, Table 2), indicating a decrease in hematite production from 300 to 350 °C. We conclude that hematite preferentially forms at 300 °C in AgPd capsules and in experiments performed with small initial grain sizes.
Charge ol350Pd10 (Table 2) combines the presence of iron oxides in amounts sufficient to be detected by XRD (Fig. 3), a very high Js (the highest of the entire dataset, Table 4), and a relatively small serpentine production (Fig. 3). Hematite is detected by XRD (Fig. 3), and the other iron oxide in the pattern is interpreted to be magnetite since magnetite (but not maghemite) has been identified in the Raman spectra of the corresponding coarse-grained charge (ol350PdG; Fig. 4). Thus, the strong Js implies a very high MMF, in fact the highest MMF in our sample set (Fig. 5b, Table 2). This indicates an exceptionally high magnetite production in this 350 °C AgPd charge performed with the smallest initial grain size.
4.2 Åheim dunite
Two experiments are available, each for internal and external control of (i.e., Au and AgPd capsules respectively) at 350 °C for similar durations (≈1050 h) and on a relatively coarse-grained starting material (≈2000 µm; Table 2). Both olivine and orthopyroxene persist in experimental products, although orthopyroxene appears more reacted. The XRD data indicate that the relative proportions of orthopyroxene decrease more markedly than olivine (Fig. 7), and dissolution textures are more clearly marked on orthopyroxene than on olivine (Fig. 2h). Serpentine appears only as fibrous chrysotile (Fig. 2h), while brucite was not found. In the Au capsules, no iron oxide could be detected either by XRD or SEM, consistent with the MMF being only slightly higher than in the starting material ( wt %; see Sect. 3.1 and Table 2). In contrast, AgPd capsule products contain an iron oxide phase detected by SEM (Fig. 2h). This iron oxide is probably responsible for the weak peaks observed on the XRD pattern (Fig. 7). No reliable Raman analysis could be obtained for this oxide, tentatively interpreted to be maghemite according to XRD pattern, knowing that this method does not allow magnetite to be clearly excluded (see Sect. 3.4). It occurs either in isolation or associated with Fe–Ni sulfides and in fractures or at grain boundaries. The measured MMF is substantially higher than in the Au capsule charge (Table 2), being also 1 order of magnitude higher than the starting material. Using a proportionality factor for maghemite of 78 A m2 kg−1 (Cullity, 1972), the increase in Js is compatible with a nominal weight of maghemite of 0.036 wt % (0.030 wt % if the iron oxide phase is magnetite). The possible occurrence of maghemite in the Åheim dunite experiment carried out in AgPd capsules is consistent with the preferential formation of hematite in the San Carlos olivine experiments carried out in AgPd. However, the cause of the polymorph change ( for hematite vs. for maghemite) remains to be clarified.
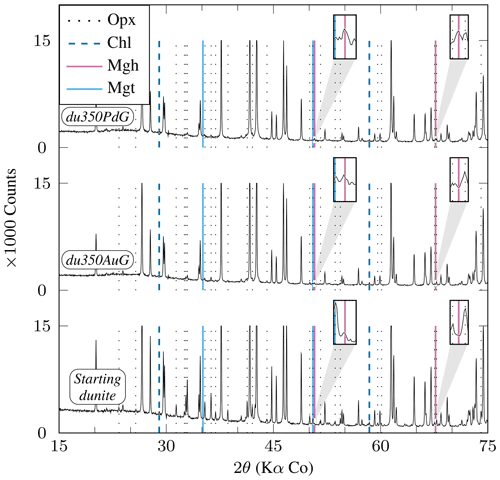
Figure 7XRD diffractograms of experimental charges produced from Åheim dunite (Table 2). Mineral abbreviations are the same as in Table 2. Peaks which are not highlighted correspond to olivine. All peaks higher than 15 000 counts are clipped. Relative peak intensities demonstrate that orthopyroxene reacts preferentially than olivine. Insets are ×6 magnifications of diffractograms centered on the 50.76° 2θ and 67.67° 2θ peaks which highlight the presence of iron oxide in du350PdG consistent with the presence of maghemite (see text).
5.1 Control of redox conditions in the experiments
Results from this study directly depend on the reliability of our methods to control the experimental redox conditions. First, it is necessary that the H2 generated by the serpentinization reaction remains confined within the Au capsule to sustain a reduced environment throughout the entire duration of the experiment. This necessitates that hydrogen loss (by permeation through the capsule, Harvie et al., 1980) is of negligible importance, despite our relatively long experimental durations. Second, H2 must escape rapidly out of the AgPd capsule so that oxidized redox conditions, controlled by the Ar pressurizing medium, are imposed to the charge. Below, we summarize the evidence confirming that both conditions were fulfilled in our experiments.
In experiments performed with Au capsules, the negligible importance of hydrogen loss is demonstrated by the following arguments:
-
Among noble metals used as capsules, hydrogen permeabilities are the lowest for Au (Chou, 1986; Gunter et al., 1987).
-
Au has been classically considered to be almost impermeable to hydrogen at low to medium temperatures, being used as the external container in double capsule assemblies used to control experimental redox states (Eugster, 1957). The permeation of hydrogen in Au is usually considered negligible below 400–450 °C (see Fig. 3 in Chou, 1986).
-
Hydrogen permeation rates have been calculated with equation 10 in Harvie et al. (1980) using Au permeability coefficients from Chou (1986) as given in Gunter et al. (1987). At 300 °C, for our capsule geometry (length: 35 mm, internal diameter: 4.6 mm, wall thickness: 0.2 mm; see above), external and internal of and 4.78 MPa respectively (Table 3), and an experimental duration of 80 d (1920 h, corresponding to the durations of our longest experiments, Table 2), a mass of 0.3 µg H2 is lost. This corresponds to <1 % of the total H2 present for capsules loaded with 100–150 mg water (Table 2). At 350 °C, the calculated hydrogen loss reaches 1 %–2 % of the total H2 present. These hydrogen losses are negligible.
-
No time-dependent hydrogen loss is apparent in our experimental results. At 300 °C, magnetite alone is present in all Au experiments, whether relatively long (ol300AuG, 2010 h) or short (ol300Au45 and ol300Au10, both ≈720 h; Table 2).
-
Experiments from the literature confirm the impermeability of Au with respect to hydrogen.
Malvoisin et al. (2012a, b) have performed serpentinization experiments of San Carlos olivine at 250–350 °C, 50 MPa for durations up to 514 d. Au capsules of 0.2 mm wall thickness and an Ar pressurizing medium were used, same as in this study. The experimental products include magnetite only, hematite being absent. Thus, there is no indication for significant hydrogen loss and for a progressive evolution towards oxidizing conditions in those experiments.
For experiments performed in AgPd capsules, the following arguments suggest that oxidized redox conditions were rapidly imposed:
-
A large contrast in hydrogen permeability constants exists between Au on the one hand and AgPd alloys on the other hand. Hydrogen permeabilities in Ag25Pd75 alloys are 8–9 orders of magnitude higher than in Au, being also higher than in pure Pd (Chou, 1986; Gunter et al., 1987).
-
Magnetite is oxidized to hematite in the A1 test experiment performed at 350 °C (Table B1 in Appendix B). In the A4 hydrogen sensor experiment, the determined ( MPa) is lower than that of the HM buffer (Table B1). Therefore, redox conditions more oxidizing than the HM buffer are recorded in the AgPd experiments at 350 °C and also presumably at 300 °C since varies little with temperature under strongly oxidizing conditions.
-
Hydrogen permeation rates for Ag25Pd75 capsules have been calculated under conditions same as above using permeability coefficients from Gunter et al. (1987). Hydrogen transfer from inside to outside the capsule is extremely fast. For external and internal of and 4.78 MPa respectively, all the H2 present inside the capsule is lost in less than 1 min. Durations of hydrogen transfer vary with the internal but remain extremely short. Therefore, Ag25Pd75 capsules cannot keep a high internal for a long time if placed in a low- external environment (Ar pressure medium), and oxidized conditions are almost instantaneously imposed to the charge.
5.2 Reaction mechanisms
5.2.1 Serpentinization
Serpentine appears in all product phase assemblages from this study (with the exception of the Åheim dunite charge in Au), and this demonstrates that a serpentinization reaction takes place in the experiments. Several serpentine-forming reactions have been proposed in the literature (Seyfried et al., 2007; Marcaillou et al., 2011; Okamoto et al., 2011; Malvoisin et al., 2012a, b; McCollom et al., 2016; Fauguerolles, 2016; Lamadrid et al., 2017; Huang et al., 2017a). The general mechanism can be conveniently described by the following global reaction:
which emphasizes that serpentine, brucite, and magnetite are the main product phases of the reaction. The α, β, γ, δ, and ϵ coefficients in Reaction (R3) depend in detail of the composition of reaction products and of the partitioning of Fe2+ and Fe3+ between product phases.
Our experimental results under conditions of internal control (Au capsule experiments) at 300 °C are broadly consistent with Reaction (R3). Olivine is consumed, and both serpentine and magnetite are produced (ol300AuG, ol300Au45, ol300Au10; Table 2). However, brucite is not present in our charges (whatever the granulometry of the starting material), in contrast with what is expected from Reaction (R3). In comparison, we note that Malvoisin et al. (2012a) reported brucite in their experiments performed under the same conditions (Au capsules and temperatures) and with the same starting material (San Carlos olivine). Several explanations have been proposed for the absence of brucite in experimental and natural serpentinites. For example, a mechanism based on precipitation of carbonates could account for the absence of brucite (Kelemen and Matter, 2008), but it is irrelevant in our case since our experimental products contain no carbonate phase. High aqueous silica activities () imposed by dissolution of local orthopyroxene or inherited by the fluids from previous encountered lithologies may also explain the absence of brucite (Frost and Beard, 2007; Frost et al., 2013; Tutolo et al., 2018). This mechanism could account for the lack of brucite in the Åheim dunite but not in the San Carlos experiments. However, contamination of silica from the agate mortar during preparation of the starting olivine powders is a possibility.
According to Reaction (R3), serpentinization of Fo91 (Mg# = 0.91) olivine yields serpentine with Mg# > 91 coexisting with brucite with Mg# < 91 (Bach et al., 2006; Malvoisin et al., 2012a; Klein et al., 2013). The distribution of Mg and Fe between serpentine and brucite determines the amount of “free” Fe which is mobilized as magnetite. For example, for the serpentine–magnetite assemblage, the Mg# of serpentine increases with increasing (decreasing ) and decreasing (Frost et al., 2013) according to the following reaction:
where the incorporation of Fe3+ in serpentine (Seyfried et al., 2007; Marcaillou et al., 2011; Andreani et al., 2013) is not considered. The transition from a serpentine–magnetite to a serpentine–brucite assemblage marks a decrease in (or increase in ) at constant (Frost et al., 2013) and could be depicted by the following reaction:
Thus, a serpentine–magnetite assemblage is indicative of conditions more oxidizing than a serpentine–brucite assemblage. In our internally controlled experiments, H2 accumulation in the Au capsule is accompanied by an increase in (and a decrease in ). However, because serpentine production is small in our experiments (see Sect. 5.2.2), did not increase (and did not decrease) sufficiently for the stabilization of a serpentine–brucite assemblage, and the system remained within the serpentine–magnetite domain in terms of and . A similar type of interpretation was proposed by Okamoto et al. (2011), who observed the appearance of brucite only after ≈500 h in hydration experiments of olivine plus water at 250 °C vapor-saturated. The late formation of brucite was attributed to a progressive evolution of the fluid from early oxidizing (i.e., sulfate reduction consumes H2) to more reducing (serpentinization leads to H2 accumulation) conditions during the experiments (Okamoto et al., 2011). For lower (and higher ) as in the externally controlled experiments in AgPd, the appearance of brucite would be even more unlikely, although we recognize that the also comes into play (see Godard et al., 2013). The presence of brucite in the experiments of Malvoisin et al. (2012b, a) could reflect conditions more reducing (higher ) than in our internally controlled experiments, which could have been promoted by globally higher serpentine production and lower ratios (up to 5 times lower) than in this study. Indeed, a lower ratio implies, for identical amounts of Fe2+ oxidized into Fe3+ and consequently for identical amounts of produced H2, a higher concentration of H2 and so a higher (Klein et al., 2013). At 350 °C, the absence of brucite is less surprising since it is actually not predicted to form at the expense of Fo90, only small amounts of serpentine and trivial amounts of magnetite being expected (Klein et al., 2009, 2013).
Reaction (R4) can also be applied to explain the banded texture of reaction products in veins within the San Carlos olivines (Fig. 2b). This texture is interpreted as a chemical zonation between an early relatively Mg-rich (dark part) and a later Fe-rich (light part) serpentine. According to Reaction (R4), the transition from the dark to the light part would be explained by growth of serpentine under more reducing (higher ) and/or higher .
For conditions of external control (AgPd capsules) at 300 °C, serpentine production is higher than in the Au, and the iron oxide phases correspond to hematite (ol300PdG, ol300Pd45, ol300Pd10), although magnetite can be still present (ol300PdG and ol300Pd10; Fig. 5a, Table 2). Thus, Reaction (R3) can be rewritten as
Reaction (R6) can be viewed as the combination of Reaction (R3) with the following reaction:
which is the HM oxygen buffer reaction. It shows that, with hematite instead of magnetite as a product phase, the proportion of Fe2+ oxidized to Fe3+ is higher than in Reaction (R3). This implies in principle that more H2 should be produced from Reaction (R6) than from Reaction (R3) (e.g., Frost, 1985). However, this conclusion needs to be confirmed in practice with the analysis of product phases in the two model Reactions (R3) and (R6). Andreani et al. (2013) have shown that, during the early stages of serpentinization, Fe3+-bearing serpentine is produced and this generates more H2 than expected from the amount of crystallized iron oxides (magnetite in their case).
5.2.2 Reaction progress
As is the case for other metasomatic processes, serpentinization is an irreversible transformation (Fonteilles, 1978). Previous experimental work has shown that serpentinization is a relatively slow reaction (Malvoisin et al., 2012a, b). Serpentine progressively forms when increasing the experimental duration, but bulk equilibrium at the scale of the whole charge (which would correspond to a 100 % reaction progress with all olivine present transformed to serpentine; see Sect. 5.2.1) is usually not reached. The advancement of the serpentinization reaction has been classically evaluated from the mass fraction of magnetite in reaction products (Oufi et al., 2002; Malvoisin et al., 2012a, b). This approach remains valid as long as magnetite is produced as the only iron oxide in the reaction. This situation occurs in our internally controlled experiments (Au capsules) at 300 °C. Using model 3 of Malvoisin et al. (2012b), which relates the amount of magnetite formed and the reaction progress (S) through the amount of water incorporated in hydrous phases (serpentine, brucite), advancements of 0.9 %, 1.3 %, and 5.4 % are obtained for ol300AuG, ol300Au45, and ol300Au10, respectively (Table 2). Thus, the serpentinization reaction is relatively little advanced in our 300 °C experiments, in agreement with the low amounts of serpentine in XRD spectra and the lack of brucite in experimental products. The S values demonstrate a progressive increase of reaction kinetics with decreasing initial grain size at 300 °C, although durations are not exactly the same between the three experiments (2010 h for ol300AuG vs. 720 h for ol300Au45 and ol300Au10; Table 2). At 350 °C in Au charges, the advancement of reaction cannot be estimated because the MMFs are not systematically higher than the baseline (see Fig. 5). Furthermore, the presence of hematite (at least in ol350AuG) excludes the use of this method. If comparison is made for similar durations and experimental parameters, reaction advancements in our 300 °C Au experiments are significantly smaller than those observed by Malvoisin et al. (2012a). For example, for a 720 h run with an initial grain size of 38–50 µm at 300 °C, an advancement of about 6 % was measured (Run #10 in Malvoisin et al., 2012a), vs. 1.3 % in this study for a similar granulometry (ol300Au45, Table 2). This stresses differences in reaction kinetics between studies, although there is also a substantial intrinsic variability between experiments. As an illustration, for different runs of 720 h duration with a 5–15 µm initial grain size at 300 °C, reaction advancements cover a large range from 13 to 70 % (Runs #16 and #22, respectively, in Malvoisin et al., 2012a). In ol300Au10 (our experiment the closest in duration and initial grain size for comparison), the advancement is 5.4 % (Table 2). This suggests the existence of rate-limiting mechanisms acting on the kinetics of the serpentinization reaction in addition to the initial granulometry. One of these mechanisms could be armoring due to precipitation of secondary phases, which limits access of the fluid to the olivine surface (Oelkers et al., 2018). This mechanism would be particularly critical if, as suggested by Malvoisin et al. (2012a), reaction kinetics are correlated to surfaces of olivine grains and the dissolution process of olivine acts as the limiting factor. Another indication for the slow serpentinization kinetics in our experiments is provided by the absence of brucite since it is symptomatic of low- conditions consistent with a small serpentine production (see above and Okamoto et al., 2011).
In our externally controlled experiments (AgPd capsules), the continuous removal of H2 from the charge toward the pressurizing medium could act to promote the advancement of the serpentinization reaction (Le Chatelier principle). Since hematite sequesters Fe, the proportion of magnetite is necessarily impacted, and models based on the production of magnetite to quantify the advancement of the serpentinization reaction are no longer applicable. However, the XRD and MMF data show that the highest production of serpentine and magnetite are reached in the externally controlled experiments (ol300Pd10 at 300 °C and ol350Pd10 at 350 °C, respectively). An enhanced advancement of the serpentinization reaction in externally controlled experiments is also consistent with ol300Pd45, the only charge with initial grain size >10 µm having serpentine and hematite detected by XRD. The increase in serpentine proportion from ol300Pd45 to ol300Pd10 (Fig. 3), i.e., upon decreasing the initial grain size, is not accompanied by an increased magnetite production since MMF values remain practically unchanged (Fig. 5a, Table 2). Therefore, in the externally controlled experiments, the advancement of the serpentinization reaction and the production of magnetite are clearly decoupled. This has important consequences for the estimation of serpentinization rates in natural oceanic systems (Cannat et al., 2010).
5.2.3 Role of temperature
Experiments performed on San Carlos olivines have demonstrated a decrease in serpentine production upon increasing temperature from 300 to 350 °C (see Sect. 4.1.4). In the same way, there is a general decrease in the MMF and magnetite production from 300 to 350 °C with the exception of one charge (ol350Pd10). Overall, these observations indicate that the serpentinization Reactions (R3) and (R6) are fully operative at 300 °C and much less developed at 350 °C, although serpentine is still present as indicated by results both on the Åheim dunite and the San Carlos olivines (Table 2). This has the consequence that less H2 is generated at 350 than at 300 °C, so reaches lower values in Au capsule experiments at 350 than at 300 °C, which explains the presence of hematite in ol350AuG (Table 2). These results are consistent with previous experimental and theoretical studies where 350 °C was found to be approximately the upper temperature limit for serpentinization (McCollom and Bach, 2009; Klein et al., 2013). Thermodynamic modeling of serpentinization reactions has shown that, for an oxidizing fluid and a high ratio, the serpentine stability field can slightly exceed 350 °C; under these conditions, the magnetite proportions increase and hematite appears as a reaction product (Malvoisin, 2015; Ely et al., 2023). Therefore, our 350 °C experimental results are qualitatively consistent with these calculations, with the exception of one AgPd charge (ol350Pd10). This 350 °C charge is characterized by a very important iron oxide production (see Sect. 4.1.4). It has a MMF much higher than in the corresponding charge at 300 °C (ol300Pd10) and which also markedly exceeds that in the corresponding Au charge at 350 °C (ol350Au10; Fig. 5; Table 2). The increase in MMF with decreasing grain size is also very abrupt in the 350 °C AgPd experiments (Fig. 5b), thus stressing the anomalous character of the charge. Since iron oxide production in ol350Pd10 is decoupled from serpentine formation (see Sect. 4.1.4), one possibility to be considered to explain the abnormally high magnetite production and presence of hematite is direct oxidation of olivine (e.g., Khisina et al., 1998; Knafelc et al., 2019). However, such mechanisms have been shown to work under anhydrous conditions and at temperatures generally higher than in this study. If operative at 350 °C under hydrothermal conditions, we note that olivine oxidation should be also observed in the 350 °C Au charge (ol350Au10), which is not the case (Fig. 5b; Table 2). Therefore, a satisfactory explanation of the ol350Au10 charge must await further studies.
5.3 Iron oxides as redox indicators in serpentinization environments
H2 production in serpentinization environments is coupled with the oxidation of Fe2+ into Fe3+ incorporated in serpentine and iron oxides (see Sect. 1). Among those, magnetite () is the most common iron oxide in serpentinites. It is also the main iron oxide produced in experimental and thermodynamic studies of serpentinization (see Sect. 1 for references). Magnetite is emblematic of the high (and low ) characteristic of serpentinization environments. This is illustrated by our internally controlled (Au capsule) experiments at 300 °C where H2 accumulates inside the capsule, leading to high- and thus low- conditions (Fig. 1a; Table 3). Magnetite forms as the main iron oxide phase (Fig. 5a; Table 2) together with serpentine according to Reaction (R3) (see Sect. 5.2.1). However, magnetite is stable in a wide range of , being limited by the hematite–magnetite (HM) and iron–magnetite (IM) reactions at high and low respectively. Note that wüstite () formation is not expected for the relatively low temperatures at which the serpentinization process operates (i.e., < 560–570 °C; Haas and Robie, 1973; Myers and Eugster, 1983). Therefore, the presence of magnetite only provides broad constraints on redox conditions in serpentinites. The occurrence of awaruite () in natural (Chamberlain et al., 1965; Frost, 1985; Klein and Bach, 2009) and possibly in experimental (McCollom et al., 2020a, b) serpentinization environments testify to the highly reductive conditions that can be reached ( according to Frost et al., 2013). Furthermore, awaruite precipitation indicates that H2-consuming reactions (which reduce part of the Fe3+ involved in magnetite formation) are also taking place. This illustrates that the of serpentinites is determined by the combination of several reactions, some acting in opposed directions.
Our externally (AgPd capsules) controlled experiments carried out at 300 °C demonstrate that hematite () appears as a reaction product of a serpentine-producing reaction (Reaction R6; see Sect. 5.2.1). The H2 generated is continuously extracted and transferred through the permeable capsule to the Ar pressurizing medium (Fig. 1a). Hematite appears in isolation, i.e., without being associated with magnetite as demonstrated by the near baseline MMF in ol300Pd45 (Fig. 5a). This, as well as the conditions above the HM buffer in the externally controlled experiments, suggests that hematite is directly formed instead of representing a product from the transformation of an earlier magnetite. Hematite formation has been expected theoretically (e.g., Frost, 1985) and hematite identified as a product phase in thermodynamic modeling of serpentinization reactions (Malvoisin, 2015; Ely et al., 2023). Hematite has also been reported in several experimental studies. It occurs in open-system reactive percolation experiments of CO2-enriched fluids in olivine aggregates and serpentinite (Peuble et al., 2019; Osselin et al., 2022). Closed-system carbonation of partially serpentinized and weathered peridotites have also yielded hematite-bearing product assemblages (Hövelmann et al., 2011). Lafay et al. (2018) observed the appearance of hematite in closed-system carbonation experiments of olivine single crystals. In contrast, parallel hydration experiments yielded only magnetite without hematite (Lafay et al., 2018). Peuble et al. (2019) assigned the oxidation of Fe2+ to the reduction of CO2 rather than of H2O as classically proposed in serpentinizing environments. For CO2-free conditions, hematite appears only in the reactive percolation study of Godard et al. (2013) performed with an oxidizing ingress fluid equilibrated with atmospheric O2. It is notably absent from the high-pressure closed-system peridotite hydration experiments of Nakatani and Nakamura (2016), despite one buffered at HM. In this particular experiment, the H2 generated in the reaction chamber was extracted (as in the present study) and consumed to oxidize hematite of the external buffer, and only magnetite formed with serpentine. We conclude that hematite formation in previous studies is a consequence of the addition of an oxidizing chemical component to the system (either CO2 or O2). In contrast, in the present study, a causal relation is demonstrated between the presence of hematite and the redox parameters. The appearance of hematite (and the transition from magnetite to hematite) is the result of imposing specific redox conditions (low and high ) during the serpentinization process.
In natural contexts, iron oxide phases other than magnetite, such as maghemite, have been reported (Prévot et al., 1981; Krammer, 1990; Nazarova et al., 2000). Hematite is also present although rarely found in the serpentinized oceanic crust (Smith and Banerjee, 1985; Bach et al., 2004; Huang et al., 2017a). When present, natural hematite has been interpreted as related to a seawater–rock interaction process that postdates the serpentinization event (Bach et al., 2004). Following the final and most advanced stages of serpentinization, fluid circulations lead to the dissolution of magnetite and its replacement by hematite as the iron oxide phase. This mechanism occurs under oxidizing conditions as indicated by the abundance of hematite in Hole 1268A and iowaite in Hole 1272A (Bach et al., 2004).
5.4 Implication for serpentinization processes at MORs
Experiments of this paper demonstrate that the H2 mobility has an influence on the serpentinization reaction. The internally controlled experiments lead to the confinement of H2, whereas, in the externally controlled experiments, H2 is extracted from the reacting serpentinization system. Contrasted redox (reducing vs. oxidizing) conditions and product phase assemblages (magnetite vs. hematite) characterize the two regimes. The experimental methodology implemented for H2 in this paper is closely analogous to the “internally controlled” vs. “externally controlled” cases classically considered in the modeling of metasomatic processes such as serpentinization (Evans et al., 2013). Below, we use the mobility of H2 as a proxy of fluid circulation, open vs. closed, in oceanic hydrothermal systems and examine the implications of fluid circulations for redox control of serpentinization processes at MORs.
In our internally controlled experiments, the is imposed by the fluid–rock interaction process (serpentinization). This situation is analogous to the one prevailing in rocks having low permeabilities and whose behavior approaches that of a “closed” system. The permeability data available for ultramafic rocks (Villeneuve et al., 2014; Farough et al., 2016; Tutolo et al., 2018) show that such closed-system situations generally apply to non-fractured lithologies. It is also worth emphasizing that, because of the volume expansion that accompanies serpentinization (Kelemen and Hirth, 2012; Evans et al., 2020; Klein and Le Roux, 2020; Malvoisin et al., 2020), porosity will tend to decrease upon hydration of ultramafic rocks, thus lowering their permeability (Tutolo et al., 2018). Ocean floor serpentinites from the MARK area record serpentinization stages formed in a closed, diffusive system, and their high serpentinization rates imply large volume expansions of the rocks (Andreani et al., 2007). Under these conditions, the fluid composition, including the H2 concentration and the , will be determined by rock-dominated fluid–rock interactions (e.g., Frost et al., 2013). It is important to emphasize that this is the case of most experimental serpentinization studies, which as a rule are performed with ratios < 3 (Seyfried et al., 2007; Malvoisin et al., 2012a, b; Malvoisin and Brunet, 2014; Klein et al., 2015; McCollom et al., 2016, 2020a, b; Fauguerolles, 2016; Table 2). These studies yield high concentrations of dissolved H2 () in the fluid, high , and low (Table 3). Small ratios are also considered some thermodynamic simulations of serpentinization (e.g., McCollom and Bach, 2009; Klein et al., 2013). Natural serpentinites have mineral assemblages that closely correspond to the experimental and thermodynamic simulations. They comprise serpentine, brucite, and magnetite as dominant phases (Mével, 2003; Bach et al., 2006; Klein et al., 2014). Properties of product phases such as magnetite (magnetic properties) are similar between natural and experimental serpentinites (Fig. 5). The common occurrence of awaruite (FeNi3) as an accessory phase (Frost, 1985; Klein and Bach, 2009; Foustoukos et al., 2015) is also consistent with a “closed” system in which conditions are kept reducing because a low permeability.
Although the preceding situation is very close to the one simulated in our serpentinization experiments performed under internal control, one important difference between natural and experimental systems is to be noted. Experiments all start from oxidized charges and low- conditions, so the H2 concentration progressively increases with time and advancement of the reaction. In contrast, in natural systems, rock-buffering through serpentine–brucite–magnetite fluid–mineral equilibria is assumed during the initial stages of serpentinization (Beard et al., 2009; Frost et al., 2013). This implies initially high and highly reducing conditions, although the influence of initial fluids of variable has been considered in some simulations (Malvoisin, 2015). This difference in initial between experimental and natural systems is considered significant. As noted earlier, it is probably responsible for the lack of brucite in our experimental product assemblages.
Our externally controlled experiments correspond to a situation where the is imposed from outside the reacting system. This situation is analogous to a serpentinization environment where the is controlled by an externally derived fluid. We assume that serpentinization is a hydration process driven by seawater and, consequently, the external fluid should have a low . It is now accepted that serpentinization takes place in an active tectonic environment where fracturing of rock and fluid infiltration are intimately associated (Andreani et al., 2007; Iyer et al., 2008; Plümper et al., 2012; Rouméjon and Cannat, 2014). For example, it has been proposed that mesh texture initiation results from tectonically controlled penetration of seawater-dominated fluids within peridotites and that the last stage of serpentinization takes place in the brittle-fracturing regime where advective fluid transfer dominates (Andreani et al., 2007; Rouméjon and Cannat, 2014). During these two stages, the fluid circulation regime is controlled by active fracturing and thus approaches an “open” system regime. In this case, certain components of the fluid phase can be externally controlled. H2 almost certainly belongs to this group given its very high mobility, but the other fluid components (e.g., ) might behave differently. Under this situation, simulated in our externally controlled experiments performed with AgPd capsules, the serpentinization reaction is modified (Reaction (R6); see Sect. 5.2.1). The appearance of hematite as a product of the reaction implies the possibility of low (high ) during serpentinization and confirms previous expectations (e.g., Frost, 1985). It is worth noting that, in thermodynamic simulations (Malvoisin, 2015; Ely et al., 2023), hematite appears only for elevated ratios (>20 and >6.5, respectively), i.e., for conditions analogous to our “open” system situation.
The near-absence of hematite in serpentinized peridotite (Smith and Banerjee, 1985; Bach et al., 2004; Huang et al., 2017a) is probably to be related to the very low conditions necessary for hematite to be stable in natural hydrothermal oceanic systems ( MPa at 300 °C; Table 1). Such low- conditions appear to be reached in specific contexts, so a serpentine–hematite–brucite assemblage can be expected to be produced in place of serpentine–magnetite–brucite usually found in serpentinization environments. However, an appropriately low- range might be rarely attained, and hematite-bearing serpentinites might be difficult to preserve since hematite would presumably react and transform if subjected to later hydrothermal stages involving higher fluids. Yet, the presence of hematite, although rare, attests to the possibility of low- conditions either locally or temporarily and, by inference, of varying during hydration of the oceanic crust. This stresses the need to consider the redox parameters as key process variables in serpentinization environments.
Our experiments highlight the key role played by the redox conditions and mechanisms of redox control during serpentinization. We have demonstrated experimentally that serpentinization can take place in a large range of (and in a correspondingly large range of ). However, although the general mechanism remains the same, notable differences were found between the internally and externally controlled experiments. At 300 °C, in Au capsules (internal control), H2 accumulation leads to a relatively high- (low-) environment characterized by the serpentine–magnetite assemblage. In comparison, in AgPd capsules (external control), continuous removal of the produced H2 yields a low- (high-) environment characterized by the serpentine–hematite(–magnetite) assemblage. At 350 °C, the lesser efficiency of the serpentinization reaction limits H2 production, and both the internally and externally controlled experiments are characterized by the serpentine–hematite(–magnetite) assemblage. The maximum serpentine production is observed at 300 °C under external control, which is in the optimum temperature range for serpentinization, the reaction being enhanced because of continuous removal of H2. Differences in product phase assemblages found in this study imply that natural serpentinization reaction mechanisms vary with redox conditions, and consequences for H2 production fluxes and rates can be expected.
The externally controlled experiments at 300 °C demonstrate that hematite can appear as a product of the serpentinization reaction. In contrast with previous studies where the presence of hematite was the consequence of the addition of an oxidizing chemical component, the present study establishes a causal relation between hematite and the imposed redox variables. The lack of brucite probably reflects conditions insufficiently reducing ( not high enough), and brucite appearance would be expected in longer internally controlled experiments (to promote reaction advancement) and for lower ratios. Brucite would be even more unlikely under low- environments as in the externally controlled experiments. We emphasize the significant difference between, on the one hand, most serpentinization experiments which start from oxidized, low- conditions and, on the other hand, natural systems where serpentinization is assumed to start at equilibrium with serpentine–brucite–magnetite and so under high .
Our experiments under external control have no counterparts among natural serpentinites mainly because these rocks typically lack hematite. However, the behavior of H2 and its differential mobility in our two types of experiments has applications for natural oceanic hydrothermal systems. At MORs, “closed-system” serpentinization stages, marked by low rock permeabilities and small ratios, alternate with serpentinization stages where “open-system” fluid infiltration is driven by tectonic activity and high transient rock permeabilities. The former fluid regime would promote a situation analogous to our experimental internal control where serpentinization leads to the production of H2 and its local accumulation. Conversely, in the latter case, the would be presumably controlled at low values by the infiltrating fluid, similar to our externally controlled experimental simulations. Given the differences noted in product phase assemblages between the two types of experiments, the possibility emerges to correlate the mineralogical composition of serpentinites with fluid circulation regimes in the oceanic crust.
The of the pressurizing gas was determined from independent experiments, all performed at 350 °C, 50 MPa, using pressure vessels, pressurizing gas, capsule materials, and durations identical to the externally -controlled serpentinization experiments (Table B1). Both indirect and direct measurements are available, the two approaches yielding globally identical results. Indirect constraints on the come from charges ran with magnetite and hematite (plus water and, in one case, San Carlos olivine). Magnetite systematically oxidized to produce either a hematite-only (Run A1) or a hematite-dominant (Run A2) product assemblage (Table B1), indicating that the imposed by the pressurizing gas is lower than that corresponding to the HM buffer ( MPa at 350 °C, 50 MPa; Table 1). Direct constraints were obtained from the hydrogen sensor method of Chou (1987) and Chou and Cygan (1990). AgCl molality of 40 mmol kg−1 was measured for the Ar pressurized vessel (Run A3), lower than that of the HM reference run (Run A4, 72 mmol kg−1 on average of A4a and A4b; Table B1) and yielding a for the pressurizing gas ( MPa) lower than that corresponding to the HM buffer ( MPa).
Table B1Experimental constraints on the of the pressurizing medium.

All experiments performed at 350 °C, 50 MPa, for 31 d, with Ar pressurizing gas and in the same pressure vessels as the serpentinization experiments. For runs A1, A2, and A4c, the starting proportion is by mass. Run A4 is the reference run (see Chou, 1987, for details of the hydrogen sensor method). Mineral abbreviations: Mgt: magnetite; Hm: hematite; Ol: San Carlos olivine.
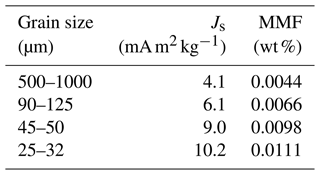
The minimum detectable MMF is approximated by the MMF of magnetite-free olivines. The Js of starting San Carlos olivines of different grain sizes has been measured (Table C1). The corresponding MMF data are fitted with a power law:
where a=0.0314, , and x is the grain size. The coefficient of determination (R2) is 0.971. This expression is used to construct the baselines in Fig. 5.
The source code for SUPCRT92 (Johnson et al., 1992) is available on Jim Palandri's (University of Oregon) website: https://pages.uoregon.edu/palandri/.
Data in Tables 2, 4, A1, A2, and A3 and in Figs. 2–5 and 7 are original data obtained by the authors for the present study. The other data used in Tables 1 and 3 and in Fig. 6 are from the literature and can be made available upon request.
CF, TC, JV, and MP designed the experiments and CF carried them out. Analytical data acquisition and processing were carried out by CF. CF and MP prepared the manuscript with contributions of TC and JV.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Probing the Earth: magma and fluids, a tribute to the career of Michel Pichavant”. It is a result of the Magma & Fluids workshop, Orléans, France, 4–6 July 2022.
We thank Claire Carvallo (IMPMC, Paris) and Erwan Le Menn (LPG, Nantes) for their help with magnetic measurements and the Raman analysis, respectively. Ida Di Carlo and Philippe Penhoud helped with the SEM and XRD.
This work forms part of Colin Fauguerolles' PhD thesis, which was supported by Région Centre, ANR (project FLUXHYD), and Labex Voltaire.
This paper was edited by Olivier Bachmann and reviewed by Carla Tiraboschi and two anonymous referees.
Abrajano, T. A., Sturchio, N. C., Kennedy, B. M., Lyon, G. L., Muehlenbachs, K., and Bohlke, J. K.: Geochemistry of reduced gas related to serpentinization of the Zambales ophiolite, Philippines, Appl. Geochem., 5, 625–630, https://doi.org/10.1016/0883-2927(90)90060-I, 1990. a
Allen, D. E. and Seyfried Jr., W. E.: Compositional controls on vent fluids from ultramafic-hosted hydrothermal systems at mid-ocean ridges: An experimental study at 400 °C, 500 bars, Geochim. Cosmochim. Ac., 67, 1531–1542, https://doi.org/10.1016/S0016-7037(02)01173-0, 2003. a, b, c, d
Andreani, M., Mével, C., Boullier, A.-M., and Escartin, J.: Dynamic control on serpentine crystallization in veins: constraints on hydration processes in oceanic peridotites, Geochem. Geophy. Geosy., 8, Q02012, https://doi.org/10.1029/2006GC001373, 2007. a, b, c, d, e
Andreani, M., Munoz, M., Marcaillou, C., and Delacour, A.: μXANES study of iron redox state in serpentine during oceanic serpentinization, Lithos, 178, 70–83, https://doi.org/10.1016/j.lithos.2013.04.008, 2013. a, b, c, d, e
Auzende, A.-L., Daniel, I., Reynard, B., Lemaire, C., and Guyot, F.: High-pressure behaviour of serpentine minerals: a Raman spectroscopic study, Phys. Chem. Miner., 31, 269–277, https://doi.org/10.1007/s00269-004-0384-0, 2004. a
Bach, W., Garrido, C. J., Paulick, H., Harvey, J., and Rosner, M.: Seawater-peridotite interactions: First insights from ODP Leg 209, MAR 15° N, Geochem. Geophy. Geosy., 5, Q09F26, https://doi.org/10.1029/2004GC000744, 2004. a, b, c, d, e
Bach, W., Paulick, H., Garrido, C. J., Ildefonse, B., Meurer, W. P., and Humphris, S. E.: Unraveling the sequence of serpentinization reactions: petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15° N (ODP Leg 209, Site 1274), Geophys. Res. Lett., 33, L13306, https://doi.org/10.1029/2006GL025681, 2006. a, b, c
Beard, J. S., Frost, B. R., Fryer, P., McCaig, A., Searle, R., Ildefonse, B., Zinin, P., and Sharma, S. K.: Onset and progression of serpentinization and magnetite formation in olivine-rich troctolite from IODP Hole U1309D, J. Petrol., 50, 387–403, https://doi.org/10.1093/petrology/egp004, 2009. a
Berckhemer, H., Kampfmann, W., Aulbach, E., and Schmeling, H.: Shear modulus and Q of forsterite and dunite near partial melting from forced-oscillation experiments, Phys. Earth Planet. In., 29, 30–41, https://doi.org/10.1016/0031-9201(82)90135-2, 1982. a
Berndt, M. E., Allen, D. E., and Seyfried Jr., W. E.: Reduction of CO2 during serpentinization of olivine at 300 °C and 500 bar, Geology, 24, 351–354, https://doi.org/10.1130/0091-7613(1996)024<0351:ROCDSO>2.3.CO;2, 1996. a, b, c, d
Burnham, C. W., Holloway, J. R., and Davis, N. F.: Thermodynamic Properties of Water to 1,000 °C and 10,000 bars, Vol. 132, Geological Society of America, https://doi.org/10.1130/SPE132, 1969. a
Cannat, M.: Emplacement of mantle rocks in the seafloor at mid-ocean ridges, J. Geophys. Res.-Sol. Ea., 98, 4163–4172, https://doi.org/10.1029/92JB02221, 1993. a
Cannat, M., Fontaine, F., and Escartín, J.: Serpentinization and associated hydrogen and methane fluxes at slow spreading ridges, in: Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, edited by: Rona, P. A., Devey, C. W., Dyment, J., and Murton, B. J., Vol. 188 of Geophysical Monograph Series, 241–264, American Geophysical Union, Washington, D.C., https://doi.org/10.1029/2008GM000760, 2010. a, b
Chamberlain, J. A., McLeod, C. R., Traill, R. J., and Lachance, G. R.: Native metals in the Muskox intrusion, Can. J. Earth Sci., 2, 188–215, https://doi.org/10.1139/e65-017, 1965. a, b
Charlou, J. L., Donval, J. P., Fouquet, Y., Jean-Baptiste, P., and Holm, N.: Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′ N, MAR), Chem. Geol., 191, 345–359, https://doi.org/10.1016/S0009-2541(02)00134-1, 2002. a
Charlou, J. L., Donval, J. P., Konn, C., Ondréas, H., Fouquet, Y., Jean-Baptiste, P., and Fourré, E.: High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge, in: Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, edited by: Rona, P. A., Devey, C. W., Dyment, J., and Murton, B. J., Vol. 188 of Geophysical Monograph Series, 265–296, American Geophysical Union, Washington, D.C., https://doi.org/10.1029/2008GM000752, 2010. a
Chou, I.-M.: Permeability of precious metals to hydrogen at 2 kb total pressure and elevated temperatures, Am. J. Sci., 286, 638–658, https://doi.org/10.2475/ajs.286.8.638, 1986. a, b, c, d
Chou, I.-M.: Oxygen buffer and hydrogen sensor techniques at elevated pressures and temperatures, in: Hydrothermal Experimental Techniques, edited by: Ulmer, G. C. and Barnes, H. L., 61–99, John Wiley and Sons, New York, NY, 1987. a, b, c, d, e
Chou, I.-M. and Cygan, G. L.: Quantitative redox control and measurement in hydrothermal experiments, in: Fluid-mineral Interactions: A Tribute to H. P. Eugster, edited by: Spencer, R. J. and Chou, I. M., Vol. 2 of Geochemical Society: Special publication, 3–15, Geochemical Society, ISBN 0-941809-01-3, 1990. a, b
Cullity, B. D.: Introduction to Magnetic Materials, Addison-Wiley Publishing Company, New Jersey, ISBN 0201012189, 1972. a
Day, R., Fuller, M., and Schmidt, V. A.: Hysteresis properties of titanomagnetites: grain-size and compositional dependence, Phys. Earth Planet. In., 13, 260–267, https://doi.org/10.1016/0031-9201(77)90108-X, 1977. a, b
de Faria, D. L. A., Venâncio Silva, S., and de Oliveira, M. T.: Raman microspectroscopy of some iron oxides and oxyhydroxides, J. Raman Spectrosc., 28, 873–878, https://doi.org/10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B, 1997. a
Drummond Jr., S. E.: Boiling and Mixing of Hydrothermal Fluids: Chemical Effects on Mineral Precipitation, PhD thesis, The Pennsylvania State University, 1981. a
Dunlop, D. J.: Theory and application of the Day plot ( versus ) 1. Theoretical curves and tests using titanomagnetite data, J. Geophys. Res.-Sol. Ea., 107, EPM 4-1–EPM 4-22, https://doi.org/10.1029/2001JB000486, 2002. a
Ely, T. D., Leong, J. M., Canovas, P. A., and Shock, E. L.: Huge variation in H2 generation during seawater alteration of ultramafic rocks, Geochem. Geophy. Geosy., 24, e2022GC010658, https://doi.org/10.1029/2022GC010658, 2023. a, b, c, d, e, f
Escartín, J., Hirth, G., and Evans, B.: Effects of serpentinization on the lithospheric strength and the style of normal faulting at slow-spreading ridges, Earth Planet. Sc. Lett., 151, 181–189, https://doi.org/10.1016/S0012-821X(97)81847-X, 1997. a
Escartín, J., Hirth, G., and Evans, B.: Strength of slightly serpentinized peridotites: Implications for the tectonics of oceanic lithosphere, Geology, 29, 1023–1026, https://doi.org/10.1130/0091-7613(2001)029<1023:SOSSPI>2.0.CO;2, 2001. a
Eugster, H. P.: Heterogeneous reactions involving oxidation and reduction at high pressures and temperatures, J. Chem. Phys., 26, 1760–1761, https://doi.org/10.1063/1.1743626, 1957. a, b
Eugster, H. P. and Skippen, G. B.: Igneous and metamorphic reactions involving gas equilibria, in: Researches in geochemistry, edited by: Abelson, P. H., Vol. 2, 492–520, John Wiley and Sons, New York, NY, 1967. a
Evans, B. W.: Control of the products of serpentinization by the exchange potential of olivine and orthopyroxene, J. Petrol., 49, 1873–1887, https://doi.org/10.1093/petrology/egn050, 2008. a, b
Evans, K. A., Powell, R., and Frost, B. R.: Using equilibrium thermodynamics in the study of metasomatic alteration, illustrated by an application to serpentinites, Lithos, 168, 67–84, https://doi.org/10.1016/j.lithos.2013.01.016, 2013. a, b, c, d
Evans, O., Spiegelman, M., and Kelemen, P. B.: Phase-field modeling of reaction-driven cracking: Determining conditions for extensive olivine serpentinization, J. Geophys. Res.-Sol. Ea., 125, e2019JB018614, https://doi.org/10.1029/2019JB018614, 2020. a, b
Farough, A., Moore, D. E., Lockner, D. A., and Lowell, R. P.: Evolution of fracture permeability of ultramafic rocks undergoing serpentinization at hydrothermal conditions: An experimental study, Geochem. Geophy. Geosy., 17, 44–55, https://doi.org/10.1002/2015GC005973, 2016. a, b
Fauguerolles, C.: Étude expérimentale de la production d'H2 associée à la serpentinisation des péridotites au niveau des dorsales océaniques lentes, Quantification – États rédox – Mécanismes réactionnels, PhD thesis, Université d'Orléans, https://theses.hal.science/tel-01549129 (last access: 27 June 2024), 2016. a, b, c, d, e, f
Fonteilles, M.: Les mécanismes de la métasomatose, B. Minéral., 101, 166–194, https://doi.org/10.3406/bulmi.1978.7185, 1978. a
Foustoukos, D. I., Bizimis, M., Frisby, C., and Shirey, S. B.: Redox controls on Ni-Fe-PGE mineralization and Re/Os fractionation during serpentinization of abyssal peridotite, Geochim. Cosmochim. Ac., 150, 11–25, https://doi.org/10.1016/j.gca.2014.11.025, 2015. a
Frost, B. R.: On the stability of sulfides, oxides, and native metals in serpentinite, J. Petrol., 26, 31–63, https://doi.org/10.1093/petrology/26.1.31, 1985. a, b, c, d, e, f, g, h, i
Frost, B. R. and Beard, J. S.: On silica activity and serpentinization, J. Petrol., 48, 1351–1368, https://doi.org/10.1093/petrology/egm021, 2007. a
Frost, B. R., Evans, K. A., Swapp, S. M., Beard, J. S., and Mothersole, F. E.: The process of serpentinization in dunite from New Caledonia, Lithos, 178, 24–39, https://doi.org/10.1016/j.lithos.2013.02.002, 2013. a, b, c, d, e, f, g, h, i
Garrels, R. M. and Thompson, M. E.: A chemical model for sea water at 25 °C and one atmosphere total pressure, Am. J. Sci., 260, 57–66, https://doi.org/10.2475/ajs.260.1.57, 1962. a
Godard, M., Luquot, L., Andreani, M., and Gouze, P.: Incipient hydration of mantle lithosphere at ridges: A reactive-percolation experiment, Earth Planet. Sc. Lett., 371, 92–102, https://doi.org/10.1016/j.epsl.2013.03.052, 2013. a, b, c, d, e, f
Gunter, W. D., Myers, J., and Girsperger, S.: Hydrogen: Metal Membranes, in: Hydrothermal Experimental Techniques, edited by: Ulmer, G. C. and Barnes, H. L., 100–120, John Wiley and Sons, New York, NY, 1987. a, b, c, d, e, f
Haas, J. L. and Robie, R. A.: Thermodynamic data for wustite, Fe0.947O, magnetite, Fe3O4, and hematite, Fe2O3, in: Transactions-American Geophysical Union, Vol. 54, p. 483, American Geophysical Union, Whashinton DC, https://doi.org/10.1029/EO054i005p00222, 1973. a, b
Harvie, C., Weare, J. H., and O'Keefe, M.: Permeation of hydrogen through platinum: A re-evaluation of the data of Chou et al, Geochim. Cosmochim. Ac., 44, 899–900, https://doi.org/10.1016/0016-7037(80)90271-9, 1980. a, b
Hövelmann, J., Austrheim, H., Beinlich, A., and Munz, I. A.: Experimental study of the carbonation of partially serpentinized and weathered peridotites, Geochim. Cosmochim. Ac., 75, 6760–6779, https://doi.org/10.1016/j.gca.2011.08.032, 2011. a, b
Huang, R., Lin, C.-T., Sun, W., Ding, X., Zhan, W., and Zhu, J.: The production of iron oxide during peridotite serpentinization: Influence of pyroxene, Geosci. Front., 8, 1311–1321, https://doi.org/10.1016/j.gsf.2017.01.001, 2017a. a, b, c, d, e
Huang, R., Song, M., Ding, X., Zhu, S., Zhan, W., and Sun, W.: Influence of pyroxene and spinel on the kinetics of peridotite serpentinization, J. Geophys. Res.-Sol. Ea., 122, 7111–7126, https://doi.org/10.1002/2017JB014231, 2017b. a
Huang, R., Sun, W., Song, M., and Ding, X.: Influence of pH on molecular hydrogen (H2) generation and reaction rates during serpentinization of peridotite and olivine, Minerals, 9, 661, https://doi.org/10.3390/min9110661, 2019. a
Iyer, K., Jamtveit, B., Mathiesen, J., Malthe-Sørenssen, A., and Feder, J.: Reaction-assisted hierarchical fracturing during serpentinization, Earth Planet. Sc. Lett., 267, 503–516, https://doi.org/10.1016/j.epsl.2007.11.060, 2008. a, b
Jackson, I., Paterson, M. S., and Gerald, J. D. F.: Seismic wave dispersion and attenuation in Åheim dunite: an experimental study, Geophys. J. Int., 108, 517–534, https://doi.org/10.1111/j.1365-246X.1992.tb04633.x, 1992. a, b
Janecky, D. R. and Seyfried, W. E.: Hydrothermal serpentinization of peridotite within the oceanic crust: experimental investigations of mineralogy and major element chemistry, Geochim. Cosmochim. Ac., 50, 1357–1378, https://doi.org/10.1016/0016-7037(86)90311-X, 1986. a
Johnson, J. W., Oelkers, E. H., and Helgeson, H. C.: SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1–5000 bar and 0–1000 °C, Comput. Geosci., 18, 899–947, https://doi.org/10.1016/0098-3004(92)90029-Q, 1992 (code available at: https://pages.uoregon.edu/palandri/, last access: 20 June 2024). a, b, c, d
Karson, J. A., Thompson, G., Humphris, S. E., Edmond, J. M., Bryan, W. B., Brown, J. R., Winters, A. T., Pockalny, R. A., Casey, J. F., Campbell, A. C., Klinkhammer, G., Palmer, M. R., Kinzler, R. J., and Sulanowska, M. M.: Along-axis variations in seafloor spreading in the MARK area, Nature, 328, 681–685, https://doi.org/10.1038/328681a0, 1987. a
Kelemen, P. B. and Hirth, G.: Reaction-driven cracking during retrograde metamorphism: Olivine hydration and carbonation, Earth Planet. Sc. Lett., 345, 81–89, https://doi.org/10.1016/j.epsl.2012.06.018, 2012. a, b
Kelemen, P. B. and Matter, J.: In situ carbonation of peridotite for CO2 storage, P. Natl. Acad. Sci. USA, 105, 17295–17300, https://doi.org/10.1073/pnas.0805794105, 2008. a
Khisina, N. R., Khramov, D. A., Kleschev, A. A., and Langer, K.: Laihunitization as a mechanism of olivine oxidation, Eur. J. Mineral., 10, 229–238, https://doi.org/10.1127/ejm/10/2/0229, 1998. a
Klein, F. and Bach, W.: Fe-Ni-Co-O-S phase relations in peridotite-seawater interactions, J. Petrol., 50, 37–59, https://doi.org/10.1093/petrology/egn071, 2009. a, b, c
Klein, F. and Le Roux, V.: Quantifying the volume increase and chemical exchange during serpentinization, Geology, 48, 552–556, https://doi.org/10.1130/G47289.1, 2020. a, b
Klein, F. and McCollom, T. M.: From serpentinization to carbonation: new insights from a CO2 injection experiment, Earth Planet. Sc. Lett., 379, 137–145, https://doi.org/10.1016/j.epsl.2013.08.017, 2013. a
Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B., and Berquó, T.: Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15° N on the Mid-Atlantic Ridge, Geochim. Cosmochim. Ac., 73, 6868–6893, https://doi.org/10.1016/j.gca.2009.08.021, 2009. a, b, c
Klein, F., Bach, W., and McCollom, T. M.: Compositional controls on hydrogen generation during serpentinization of ultramafic rocks, Lithos, 178, 55–69, https://doi.org/10.1016/j.lithos.2013.03.008, 2013. a, b, c, d, e, f, g, h, i
Klein, F., Bach, W., Humphris, S. E., Kahl, W.-A., Jöns, N., Moskowitz, B., and Berquó, T. S.: Magnetite in seafloor serpentinite – Some like it hot, Geology, 42, 135–138, https://doi.org/10.1130/G35068.1, 2014. a, b, c
Klein, F., Grozeva, N. G., Seewald, J. S., McCollom, T. M., Humphris, S. E., Moskowitz, B., Berquó, T. S., and Kahl, W.-A.: Fluids in the crust. Experimental constraints on fluid-rock reactions during incipient serpentinization of harzburgite, Am. Mineral., 100, 991–1002, https://doi.org/10.2138/am-2015-5112, 2015. a, b, c
Klein, F., Grozeva, N. G., and Seewald, J. S.: Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions, P. Natl. Acad. Sci. USA, 116, 17666–17672, https://doi.org/10.1073/pnas.1907871116, 2019. a
Knafelc, J., Filiberto, J., Ferré, E. C., Conder, J. A., Costello, L., Crandall, J. R., Dyar, M. D., Friedman, S. A., Hummer, D. R., and Schwenzer, S. P.: The effect of oxidation on the mineralogy and magnetic properties of olivine, Am. Mineral., 104, 694–702, https://doi.org/10.2138/am-2019-6829, 2019. a
Korzhinskii, D. S.: The theory of systems with perfectly mobile components and processes of mineral formation, Am. J. Sci., 263, 193–205, https://doi.org/10.2475/ajs.263.3.193, 1965. a
Krammer, K.: Rock magnetic properties and opaque mineralogy of selected samples from Hole 670A, in: Proceedings of the Ocean Drilling Program, Scientific Results, edited by: Detrick, R., Honnorez, J., Bryan, W. B., Juteau, T., and et al., Vol. 106/109, College Station, TX (Ocean Drilling Program), 269–273, https://doi.org/10.2973/odp.proc.sr.106109.154.1990, 1990. a
Lafay, R., Montes-Hernandez, G., Janots, E., Chiriac, R., Findling, N., and Toche, F.: Mineral replacement rate of olivine by chrysotile and brucite under high alkaline conditions, J. Crystal Growth, 347, 62–72, https://doi.org/10.1016/j.jcrysgro.2012.02.040, 2012. a
Lafay, R., Montes-Hernandez, G., Renard, F., and Vonlanthen, P.: Intracrystalline reaction-induced cracking in olivine evidenced by hydration and carbonation experiments, Minerals, 8, 412, https://doi.org/10.3390/min8090412, 2018. a, b, c
Lamadrid, H. M., Rimstidt, J. D., Schwarzenbach, E. M., Klein, F., Ulrich, S., Dolocan, A., and Bodnar, R. J.: Effect of water activity on rates of serpentinization of olivine, Nat. Commun., 8, 16107, https://doi.org/10.1038/ncomms16107, 2017. a, b, c
Lamadrid, H. M., Zajacz, Z., Klein, F., and Bodnar, R. J.: Synthetic fluid inclusions XXIII. Effect of temperature and fluid composition on rates of serpentinization of olivine, Geochim. Cosmochim. Ac., 292, 285–308, https://doi.org/10.1016/j.gca.2020.08.009, 2021. a, b
Lazar, C.: Using silica activity to model redox-dependent fluid compositions in serpentinites from 100 to 700 °C and from 1 to 20 kbar, J. Petrol., 61, egaa101, https://doi.org/10.1093/petrology/egaa101, 2020. a
Legodi, M. A. and de Waal, D.: The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste, Dyes Pigments, 74, 161–168, https://doi.org/10.1016/j.dyepig.2006.01.038, 2007. a, b
Lemaire, C.: Application des Spectroscopies Vibrationnelles à la Détection d'Amiante dans les Matériaux et à l'Étude des Serpentines, PhD thesis, Paris 7, 2000. a
Maffione, M., Morris, A., Plümper, O., and van Hinsbergen, D. J. J.: Magnetic properties of variably serpentinized peridotites and their implication for the evolution of oceanic core complexes, Geochem. Geophy. Geosy., 15, 923–944, https://doi.org/10.1002/2013GC004993, 2014. a, b
Malvoisin, B.: Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical, Earth Planet. Sc. Lett., 430, 75–85, https://doi.org/10.1016/j.epsl.2015.07.043, 2015. a, b, c, d, e, f, g
Malvoisin, B. and Brunet, F.: Water diffusion-transport in a synthetic dunite: Consequences for oceanic peridotite serpentinization, Earth Planet. Sc. Lett., 403, 263–272, https://doi.org/10.1016/j.epsl.2014.07.004, 2014. a, b
Malvoisin, B., Brunet, F., Carlut, J., Rouméjon, S., and Cannat, M.: Serpentinization of oceanic peridotites: 2. Kinetics and processes of San Carlos olivine hydrothermal alteration, J. Geophys. Res.-Sol. Ea., 117, B04102, https://doi.org/10.1029/2011JB008842, 2012a. a, b, c, d, e, f, g, h, i, j, k, l, m, n
Malvoisin, B., Carlut, J., and Brunet, F.: Serpentinization of oceanic peridotites: 1. A high-sensitivity method to monitor magnetite production in hydrothermal experiments, J. Geophys. Res.-Sol. Ea., 117, B01104, https://doi.org/10.1029/2011JB008612, 2012b. a, b, c, d, e, f, g, h, i, j, k, l, m, n, o
Malvoisin, B., Zhang, C., Müntener, O., Baumgartner, L. P., Kelemen, P. B., and ODP Science Party: Measurement of volume change and mass transfer during serpentinization: Insights from the Oman Drilling Project, J. Geophys. Res.-Sol. Ea., 125, e2019JB018877, https://doi.org/10.1029/2019JB018877, 2020. a, b
Marcaillou, C., Munoz, M., Vidal, O., Parra, T., and Harfouche, M.: Mineralogical evidence for H2 degassing during serpentinization at 300 °C/300 bar, Earth Planet. Sc. Lett., 303, 281–290, https://doi.org/10.1016/j.epsl.2011.01.006, 2011. a, b, c, d, e
Martin, B. and Fyfe, W. S.: Some experimental and theoretical observations on the kinetics of hydration reactions with particular reference to serpentinization, Chem. Geol., 6, 185–202, https://doi.org/10.1016/0009-2541(70)90018-5, 1970. a
Mayhew, L. E., Ellison, E. T., McCollom, T. M., Trainor, T. P., and Templeton, A. S.: Hydrogen generation from low-temperature water-rock reactions, Nat. Geosci., 6, 478–484, https://doi.org/10.1038/ngeo1825, 2013. a
McCollom, T. M. and Bach, W.: Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks, Geochim. Cosmochim. Ac., 73, 856–875, https://doi.org/10.1016/j.gca.2008.10.032, 2009. a, b, c
McCollom, T. M. and Seewald, J. S.: A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine, Geochim. Cosmochim. Ac., 65, 3769–3778, https://doi.org/10.1016/S0016-7037(01)00655-X, 2001. a
McCollom, T. M., Klein, F., Robbins, M., Moskowitz, B., Berquó, T. S., Jöns, N., Bach, W., and Templeton, A.: Temperature trends for reaction rates, hydrogen generation, and partitioning of iron during experimental serpentinization of olivine, Geochim. Cosmochim. Ac., 181, 175–200, https://doi.org/10.1016/j.gca.2016.03.002, 2016. a, b, c, d, e, f, g
McCollom, T. M., Klein, F., Moskowitz, B., Berquó, T. S., Bach, W., and Templeton, A. S.: Hydrogen generation and iron partitioning during experimental serpentinization of an olivine-pyroxene mixture, Geochim. Cosmochim. Ac., 282, 55–75, https://doi.org/10.1016/j.gca.2020.05.016, 2020a. a, b, c
McCollom, T. M., Klein, F., Solheid, P., and Moskowitz, B.: The effect of pH on rates of reaction and hydrogen generation during serpentinization, Philos. T. R. Soc. A, 378, 20180428, https://doi.org/10.1098/rsta.2018.0428, 2020b. a, b, c
Mével, C.: Serpentinization of abyssal peridotites at mid-ocean ridges, C.R. Geosci., 335, 825–852, https://doi.org/10.1016/j.crte.2003.08.006, 2003. a, b
Moody, J. B.: An experimental study on the serpentinization of iron-bearing olivines, Can. Mineral., 14, 462–478, 1976. a
Myers, J. and Eugster, H. P.: The system Fe-Si-O: Oxygen buffer calibrations to 1,500 K, Contrib. Mineral. Petr., 82, 75–90, https://doi.org/10.1007/BF00371177, 1983. a, b
Nakatani, T. and Nakamura, M.: Experimental constraints on the serpentinization rate of fore-arc peridotites: Implications for the upwelling condition of the slab-derived fluid, Geochem. Geophy. Geosy., 17, 3393–3419, https://doi.org/10.1002/2016GC006295, 2016. a
Nazarova, K. A., Wasilewski, P. J., and Dick, H. J. B.: Magnetic study of serpentinized harzburgites from the Islas Orcadas Fracture Zone, Mar. Geophys. Res., 21, 475–488, https://doi.org/10.1023/A:1026550011802, 2000. a
Neal, C. and Stanger, G.: Hydrogen generation from mantle source rocks in Oman, Earth Planet. Sc. Lett., 66, 315–320, https://doi.org/10.1016/0012-821X(83)90144-9, 1983. a
Oelkers, E. H., Declercq, J., Saldi, G. D., Gislason, S. R., and Schott, J.: Olivine dissolution rates: A critical review, Chem. Geol., 500, 1–19, https://doi.org/10.1016/j.chemgeo.2018.10.008, 2018. a
Ogasawara, Y., Okamoto, A., Hirano, N., and Tsuchiya, N.: Coupled reactions and silica diffusion during serpentinization, Geochim. Cosmochim. Ac., 119, 212–230, https://doi.org/10.1016/j.gca.2013.06.001, 2013. a
Okamoto, A., Ogasawara, Y., Ogawa, Y., and Tsuchiya, N.: Progress of hydration reactions in olivine-H2O and orthopyroxenite-H2O systems at 250 °C and vapor-saturated pressure, Chem. Geol., 289, 245–255, https://doi.org/10.1016/j.chemgeo.2011.08.007, 2011. a, b, c, d, e
Osselin, F., Pichavant, M., Champallier, R., Ulrich, M., and Raimbourg, H.: Reactive transport experiments of coupled carbonation and serpentinization in a natural serpentinite. Implication for hydrogen production and carbon geological storage, Geochim. Cosmochim. Ac., 318, 165–189, https://doi.org/10.1016/j.gca.2021.11.039, 2022. a, b
Oufi, O., Cannat, M., and Horen, H.: Magnetic properties of variably serpentinized abyssal peridotites, J. Geophys. Res.-Sol. Ea., 107, EPM 3-1–EPM 3-19, https://doi.org/10.1029/2001JB000549, 2002. a, b, c, d, e, f
Oyanagi, R., Okamoto, A., and Tsuchiya, N.: Silica controls on hydration kinetics during serpentinization of olivine: Insights from hydrothermal experiments and a reactive transport model, Geochim. Cosmochim. Ac., 270, 21–42, https://doi.org/10.1016/j.gca.2019.11.017, 2020. a, b
Peuble, S., Godard, M., Gouze, P., Leprovost, R., Martinez, I., and Shilobreeva, S.: Control of CO2 on flow and reaction paths in olivine-dominated basements: An experimental study, Geochim. Cosmochim. Ac., 252, 16–38, https://doi.org/10.1016/j.gca.2019.02.007, 2019. a, b, c
Pichavant, M.: Effects of B and H2O on liquidus phase relations in the haplogranite system at 1 kbar, Am. Mineral., 72, 1056–1070, 1987. a
Plümper, O., Røyne, A., Magrasó, A., and Jamtveit, B.: The interface-scale mechanism of reaction-induced fracturing during serpentinization, Geology, 40, 1103–1106, https://doi.org/10.1130/G33390.1, 2012. a, b
Prévot, M., Lecaille, A., and Mankinen, E. A.: Magnetic effects of maghemitization of oceanic crust, J. Geophys. Res.-Sol. Ea., 86, 4009–4020, https://doi.org/10.1029/JB086iB05p04009, 1981. a
Rouméjon, S. and Cannat, M.: Serpentinization of mantle-derived peridotites at mid-ocean ridges: Mesh texture development in the context of tectonic exhumation, Geochem. Geophy. Geosy., 15, 2354–2379, https://doi.org/10.1002/2013GC005148, 2014. a, b, c
Rouméjon, S., Cannat, M., Agrinier, P., Godard, M., and Andreani, M.: Serpentinization and fluid pathways in tectonically exhumed peridotites from the Southwest Indian Ridge (62–65° E), J. Petrol., 56, 703–734, https://doi.org/10.1093/petrology/egv014, 2015. a
rruff.info: Magnetite R061111, https://rruff.info/)/R061111, last access: 11 August 2023. a
Rudert, V., Chou, I.-M., and Eugster, H. P.: Temperature gradients in rapid-quench cold-seal pressure vessels, Am. Mineral., 61, 1012–1015, 1976. a
Scaillet, B., Pichavant, M., Roux, J., Humbert, G., and Lefevre, A.: Improvements of the Shaw membrane technique for measurement and control of at high temperatures and pressures, Am. Mineral., 77, 647–655, 1992. a, b
Schmidt, B. C., Scaillet, B., and Holtz, F.: Accurate control of in cold-seal pressure vessels with the Shaw membrane technique, Eur. J. Mineral., 7, 893–904, https://doi.org/10.1127/ejm/7/4/0893, 1995. a, b
Schwartz, S., Guillot, S., Reynard, B., Lafay, R., Debret, B., Nicollet, C., Lanari, P., and Auzende, A. L.: Pressure–temperature estimates of the lizardite/antigorite transition in high pressure serpentinites, Lithos, 178, 197–210, https://doi.org/10.1016/j.lithos.2012.11.023, 2013. a
Seyfried Jr., W. E. and Dibble Jr., W. E.: Seawater-peridotite interaction at 300 °C and 500 bars: implications for the origin of oceanic serpentinites, Geochim. Cosmochim. Ac., 44, 309–321, https://doi.org/10.1016/0016-7037(80)90139-8, 1980. a
Seyfried Jr., W. E., Foustoukos, D. I., and Fu, Q.: Redox evolution and mass transfer during serpentinization: An experimental and theoretical study at 200 °C, 500 bar with implications for ultramafic-hosted hydrothermal systems at Mid-Ocean Ridges, Geochim. Cosmochim. Ac., 71, 3872–3886, https://doi.org/10.1016/j.gca.2007.05.015, 2007. a, b, c, d, e
Seyfried Jr., W. E., Pester, N. J., Ding, K., and Rough, M.: Vent fluid chemistry of the Rainbow hydrothermal system (36° N, MAR): Phase equilibria and in situ pH controls on subseafloor alteration processes, Geochim. Cosmochim. Ac., 75, 1574–1593, https://doi.org/10.1016/j.gca.2011.01.001, 2011. a
Seyfried Jr., W. E., Pester, N. J., Tutolo, B. M., and Ding, K.: The Lost City hydrothermal system: Constraints imposed by vent fluid chemistry and reaction path models on subseafloor heat and mass transfer processes, Geochim. Cosmochim. Ac., 163, 59–79, https://doi.org/10.1016/j.gca.2015.04.040, 2015. a
Shaw, H. R.: Hydrogen-water vapor mixtures: control of hydrothermal atmospheres by hydrogen osmosis, Science, 139, 1220–1222, https://doi.org/10.1126/science.139.3560.1220, 1963. a
Shaw, H. R. and Wones, D. R.: Fugacity coefficients for hydrogen gas between 0 degrees and 1000 degrees C, for pressures to 3000 atm, Am. J. Sci., 262, 918–929, https://doi.org/10.2475/ajs.262.7.918, 1964. a
Sleep, N. H., Meibom, A., Fridriksson, T., Coleman, R. G., and Bird, D. K.: H2−rich fluids from serpentinization: geochemical and biotic implications, P. Natl. Acad. Sci. USA, 101, 12818–12823, https://doi.org/10.1073/pnas.0405289101, 2004. a
Smith, G. M. and Banerjee, S. K.: Magnetic-properties of plutonic rocks from the central North-Atlantic Ocean, in: Initial Reports of the Deep Sea Drilling Project, edited by: Bougault, H., Cande, S. C., Brannon, J. C., Christie, D. M., Clark, M., Curtis, D. M., Drake, N., Echols, D., Ashley Hill, I., Javed Khan, M., Mills, W., Neuser, R., Rideout, M. L., and Weaver, B. L., Vol. 82, 377–383, U.S. Government Printing Office, Washington, D.C., USA, https://doi.org/10.2973/dsdp.proc.82.117.1985, 1985. a, b
Steefel, C. I., DePaolo, D. J., and Lichtner, P. C.: Reactive transport modeling: An essential tool and a new research approach for the Earth sciences, Earth Planet. Sc. Lett., 240, 539–558, https://doi.org/10.1016/j.epsl.2005.09.017, 2005. a
Syverson, D. D., Tutolo, B. M., Borrok, D. M., and Seyfried Jr., W. E.: Serpentinization of olivine at 300 °C and 500 bars: an experimental study examining the role of silica on the reaction path and oxidation state of iron, Chem. Geol., 475, 122–134, https://doi.org/10.1016/j.chemgeo.2017.11.006, 2017. a, b
Toft, P. B., Arkani-Hamed, J., and Haggerty, S. E.: The effects of serpentinization on density and magnetic susceptibility: a petrophysical model, Phys. Earth Planet. In., 65, 137–157, https://doi.org/10.1016/0031-9201(90)90082-9, 1990. a, b
Tutolo, B. M., Luhmann, A. J., Tosca, N. J., and Seyfried Jr., W. E.: Serpentinization as a reactive transport process: the brucite silicification reaction, Earth Planet. Sc. Lett., 484, 385–395, https://doi.org/10.1016/j.epsl.2017.12.029, 2018. a, b, c, d, e, f
Villeneuve, J., Fauguerolles, C., Castelain, T., and Pichavant, M.: Étude expérimentale de l'évolution de la perméabilité des péridotites lors de la serpentinisation, 24ème Réunion des Sciences de la Terre, Pau, France, https://rst2014-pau.sciencesconf.org/conference/rst2014-pau/rstabstractsnum.pdf (last access: 27 June 2024), 2014. a
Wegner, W. W. and Ernst, W. G.: Experimentally determined hydration and dehydration reaction rates in the system MgO-SiO2-H2O, in: Studies in Metamorphism and Metasomatism, edited by: Greenwood, H. J., Vol. 283-A, 151–180, American Journal of Science, New Haven, Connecticut, 1983. a
Weill, D. F. and Fyfe, W. S.: A discussion of the Korzhinskii and Thompson treatment of thermodynamic equilibrium in open systems, Geochim. Cosmochim. Ac., 28, 565–576, https://doi.org/10.1016/0016-7037(64)90077-8, 1964. a
- Abstract
- Introduction
- Background
- Materials and methods
- Results
- Discussion
- Conclusions
- Appendix A: Starting material composition
- Appendix B: of Ar pressurizing medium
- Appendix C: Determination of MMF baseline
- Code availability
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Abstract
- Introduction
- Background
- Materials and methods
- Results
- Discussion
- Conclusions
- Appendix A: Starting material composition
- Appendix B: of Ar pressurizing medium
- Appendix C: Determination of MMF baseline
- Code availability
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References