the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mckelveyite group minerals – Part 2: Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, a new species
Inna Lykova
Ralph Rowe
Glenn Poirier
Henrik Friis
Kate Helwig
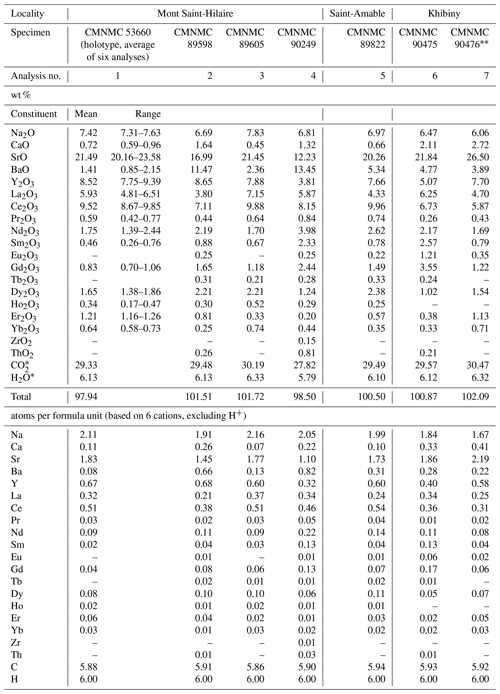
The new mckelveyite group mineral alicewilsonite-(YCe), ideally Na2Sr2YCe(CO3)6 ⋅ 3H2O, was found at Mont Saint-Hilaire, Quebec, Canada, and subsequently at the Saint-Amable sill, Quebec, Canada, and the Khibiny Massif, Kola Peninsula, Russia. Alicewilsonite-(YCe) crystals are commonly hemimorphic pseudotrigonal and pseudohexagonal and show barrel-shaped, saucer-shaped, spindle-shaped, cone-shaped, columnar, tabular, and platy habits. They are usually up to 2–3 mm in size with some larger crystals reaching 2–3 cm. The crystals often form stacked or parallel growth aggregates and rosettes. Alicewilsonite-(YCe) colour varies from pale yellow to yellow, lemon yellow, green yellow, orange-yellow, pale green to green, pale grey to grey, green grey, beige, and colourless. The streak is white; the lustre is vitreous. The cleavage is fair to indistinct, parallel to (001). The Mohs hardness is 3. Dcalc is 3.37 g cm−3. Alicewilsonite-(YCe) is optically biaxial (+), with α=1.554(3), β=1.558(3), γ=1.644(2), 2V (calc.) = 26∘, 2V (meas.) = 20(3)∘ (589 nm). The IR spectrum is reported. The composition (wt %, average of six analyses) is Na2O 7.42, CaO 0.72, SrO 21.49, BaO 1.41, Y2O3 8.52, La2O3 5.93, Ce2O3 9.52, Pr2O3 0.59, Nd2O3 1.75, Sm2O3 0.46, Gd2O3 0.83, Dy2O3 1.65, Ho2O3 0.34, Er2O3 1.21, Yb2O3 0.64, CO2 29.33, H2O 6.13, total 97.94. The empirical formula of the holotype calculated on the basis of six cations is Na2.11Ca0.11Sr1.83Ba0.08Y0.67(Ce0.51La0.32Pr0.03Nd0.09Sm0.02Gd0.04 Dy0.08Ho0.02Er0.06Yb0.03)Σ1.20(CO3)5.88 (H2O)3.00. The mineral is triclinic, P1, a=9.0036(6) Å, b=9.0175(6) Å, c=6.7712(5) Å, α=102.724(2)∘, β=116.398(2)∘, γ=60.003(2)∘, V=426.46(5) Å3, and Z=1. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are 6.07(31)(001), 4.372(100)(120, 1, 10), 4.037(25)(11, 1, 210), 3.201(25)(121, 2, 11), 2.831(67)(12, 2, 211, 21, 20), 2.601(39)(030, 1,01), 2.236(24)(1, 21, 1). 2.019(23)(003, 22, , 420). 1.9742(24)(032, 03, 3, 331, 02, 301). The crystal structure, solved and refined from single-crystal X-ray diffraction data (R1=0.055), is of the weloganite type.
- Article
(11522 KB) - Full-text XML
- Companion paper 1
- Companion paper 3
- Companion paper 4
-
Supplement
(479 KB) - BibTeX
- EndNote
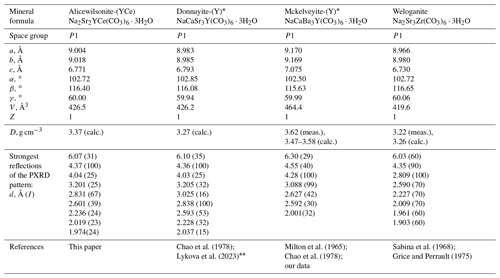
Mckelveyite group minerals are carbonates with the general formula A3B3(CO3)6 ⋅ 3H2O, where A = Na, Ca, Y, and Zr, and B = Sr, Ba, Ce, and La (Lykova et al., 2023). Seven group members are known today: mckelveyite-(Y), NaCaBa3Y(CO3)6 ⋅ 3H2O (Milton et al., 1965; Demartin et al., 2008), donnayite-(Y), NaCaSr3Y(CO3)6 ⋅ 3H2O (Chao et al., 1978), ewaldite, BaCa(CO3)2 ⋅ 2.6H2O (Donnay et al., 1971; Voloshin et al., 1992), weloganite, Na2Sr3Zr(CO3)6 ⋅ 3H2O (Sabina et al., 1968), and recently decribed bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O (IMA 2020-065); alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O (IMA 2021-047); and alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O. The latter is described in this paper.
Alicewilsonite-(YCe) is named in honour of the prominent Canadian geologist Alice Wilson (1881–1964), best known for her studies of the rocks and fossils of Ontario and Quebec (Canada). She worked at the Geological Survey of Canada for many years and taught at Carleton College (now Carleton University). She was also a Fellow of The Royal Canadian Geographical Society and the Royal Society of Canada. A parenthesised suffix is added in accordance with the nomenclature for rare-earth and Y mineral species established by Levinson (1966). The later extension of the nomenclature clarified that “only the dominant element in a specified crystal-structure site within isostructural minerals may be appended to the group name as a parenthesised suffix. This nomenclature system is mandatory for rare-earth mineral species. More than one chemical symbol may be appended only if the elements occupy different crystal-structure sites, which must be defined” (Bayliss and Levinson, 1988). Y and Ce are the dominant elements at the two non-equivalent sites in the structure of alicewilsonite-(YCe), and so two symbols are appended to the name. Based on the recently approved nomenclature of the mckelveyite group, the first symbol represents the dominant cations at one of the A sites and the second symbol at one of the B sites (Lykova et al., 2023). Therefore, the appended suffix is -(YCe).
Both the new mineral and the name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA CNMNC), proposal IMA 2020-055. The holotype of alicewilsonite-(YCe) is the specimen with the catalogue number CMNMC 53660 from the collection of the Canadian Museum of Nature, Canada, originally labelled “donnayite-(Y)”.
Alicewilsonite-(YCe) is an unnamed mineral UM1990-13-CO:HNaREESrY, also known as MSH UK-33A (Chao et al., 1990; Horvath et al., 2019). It was first marked as an unknown phase from the famous Mont Saint-Hilaire alkaline complex (Canada) around 1980 by George Chao, who worked on it for the next decade. However, our recent re-examination of donnayite-(Y) type specimens showed that data for the original description of donnayite-(Y) by Chao et al. (1978) were collected on two different species – donnayite-(Y) and alicewilsonite-(YCe) – and six out of seven cotype specimens turned out to be alicewilsonite-(YCe) (Lykova et al, 2023). Therefore, both donnayite-(Y) and alicewilsonite-(YCe) specimens were likely first collected in the 1960s and later analysed and identified as a potentially new species by Chao in the 1970s who then described donnayite-(Y) using data collected on the two species. Alicewilsonite-(YCe) was later studied by Andrew McDonald, but the species had never been formally described (Horvath et al., 2019). The major challenge with studying the mckelveyite group minerals is a poor quality of their crystals, largely unsuitable for structural analysis.
A microprobe analysis of alicewilsonite-(YCe) was published by Horvath et al. (2019) under the name UK-33A, and their results are very similar to our data. Crystal structures of two “donnayite-(Y)” polymorphs – triclinic P1 and trigonal R3m – reported by Thi et al. (1984, 1992) were also likely done on alicewilsonite-(YCe) as no Ca was reported in either structure. Petersen et al. (2003) described a REE (rare-earth element)-rich phase “similar in composition to UK-33A” in the Narssârssuk pegmatite, Igaliko alkaline complex, South Greenland. It forms one of zones in cone-shaped crystals made of ewaldite, donnayite-(Y), UK-33A, and an unknown Y and REE-rich phase. No analytical data on UK-33A were given.
Mckelveyite group minerals are typical late-stage minerals at the Poudrette (Demix) quarry, Mont Saint-Hilaire, Quebec, Canada, found in miarolitic cavities in nepheline and sodalite syenite, alkaline pegmatites, carbonate pegmatites, and marble xenoliths (Horvath et al., 2019, and the references therein). Alicewilsonite-(YCe) is the most common mckelveyite group mineral at the locality.

Figure 1Alicewilsonite-(YCe) from Mont Saint-Hilaire, Quebec, Canada: (a) green-yellow spindle-shaped prismatic crystal 2 cm in length, partially encrusted by pyrite, on albite. Alicewilsonite-(YCe) composes the outer part of the crystal, while the inner part is made of donnayite-(Y) and gaidonnayite. Specimen CMNMC 53660 (the holotype); (b) yellow saucer-shaped crystal 0.5 mm in size with brown stilpnomelane. Specimen CMNMC 89598; (c) yellow transparent platy crystals. FOV 4 mm. Specimen CMNMC 89605; (d) pale-grey stacked saucer-shaped crystals. FOV 1.5 mm. Specimen CMNMC 90249. Canadian Museum of Nature collection. (b–d) Photos: François Génier.
Mckelveyite group minerals are visually indistinguishable from each other, and most alicewilsonite-(YCe) specimens can be found in collections under names “donnayite-(Y)” or “donnayite-like”. Alicewilsonite-(YCe) crystals are most often hemimorphic pseudotrigonal and pseudohexagonal and show a wide range of habits, including barrel-shaped, saucer-shaped, spindle-shaped, cone-shaped, columnar, tabular, and platy (Fig. 1). In most cases the crystal faces are curved, split, and/or stepped. Striation parallel to (001) is common. The crystals usually reach up to 2–3 mm in size, but some larger crystals up to 2–3 cm have been also found. The crystals often form stacked or parallel growth aggregates and rosettes.
Epitaxy of alicewilsonite-(YCe) and other mckelveyite group minerals is very common (Donnay et al., 1971; Chao et al., 1978; Petersen et al, 2003; Pekov and Podlesnyi, 2004; our data). We found epitactic overgrowths of alicewilsonite-(YCe) on donnayite-(Y) and vice versa. Similar relationships were observed between alicewilsonite-(YCe) and the recently approved new species bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O. On one specimen, alicewilsonite-(YCe) was found forming an epitactic overgrowth on weloganite, Na2Sr3Zr(CO3)6 ⋅ 3H2O. Homoepitactic growth is also common.
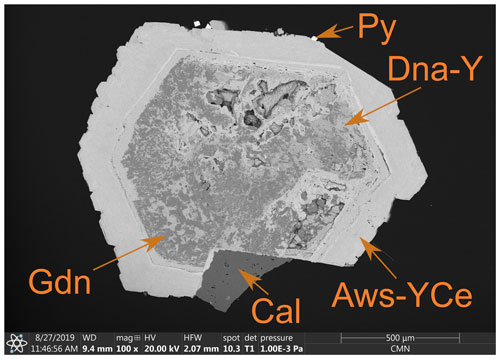
Figure 2Donnayite-(Y) partially replaced by gaidonnayite with a rim of alicewilsonite-(YCe). Aws-YCe – alicewilsonite-(YCe), Dna-Y – donnayite-(Y), Gdn – gaidonnayite, Cal – calcite, Py – pyrite. Polished section. SEM (BSE) image. Specimen CMNMC 53660 (the holotype).
The holotype specimen was collected by Gilles Haineault in the winter of 1987–1988 in a large altered alkaline pegmatite in nepheline syenite (Horvath et al., 2019). Alicewilsonite-(YCe) occurs with donnayite-(Y), gaidonnayite, albite, calcite, and pyrite. It forms rims of barrel-shaped and spindle-shaped prismatic crystals up to 3 cm in size. The crystals are hemimorphic, with curved pseudohexagonal pyramidal faces converging on one end and terminated with a pedion on the other (Fig. 1a). The core of the crystals is made of donnayite-(Y) and gaidonnayite (Fig. 2), with the latter possibly replacing the former. Alicewilsonite-(YCe) forms epitactic overgrowths on donnayite-(Y). The crystals are partially encrusted by pyrite.
We analysed a series of “donnayite-like” specimens from two other alkaline complexes: the Saint-Amable sill, Quebec, Canada, and the Khibiny Massif, Kola Peninsula, Russia. Donnayite-(Y) appears to be the most common mckelveyite group mineral at both localities, but alicewilsonite-(YCe) is also present.

Figure 3Alicewilsonite-(YCe): (a) orange-yellow transparent crystals from the Demix-Varennes quarry, Saint-Amable sill, Quebec, Canada. FOV 1.06 mm. Specimen CMNMC 89822. Photo: Michael Bainbridge and (b) green-grey crystals from the Kirovskii mine, Kukisvumchorr mountains, Khibiny Massif, Kola Peninsula, Russia. FOV 1.5 mm. Specimen CMNMC 90475. Photo: François Génier. Canadian Museum of Nature collection.
At the Demix-Varennes quarry, Saint-Amable sill (Horvath et al., 1998) alicewilsonite-(YCe) occurs as yellow to orange-yellow transparent crystals up to 0.5 mm in size (Fig. 3a) or as very thin rims around donnayite-(Y) in yellow transparent hemimorphic crystals in miarolitic cavities in nepheline syenite.
In the Hilairitovoye pegmatite at the Kirovskii mine, Kukisvumchorr mountains, Khibiny Massif (Pekov and Podlesnyi, 2004), alicewilsonite-(YCe) forms zoned green-grey tabular crystals up to 0.3 mm in size (Fig. 3b). It seemingly both replaced and epitactically overgrew donnayite-(Y), which is partially preserved in the core of the crystals. The material was collected by Alexander Podlesnyi, and the main specimen is currently in the collection of Igor Pekov, no. 7421, while the analysed fragment was deposited at the Canadian Museum of Nature, specimen CMNMC 90475. Alicewilsonite-(YCe) was also found on another specimen collected by Alexander Podlesnyi at the Kirovskii mine in the early 1980s. It forms the outer zone of yellow transparent “caps” on white opaque donnayite-(Y) crystals, whereas the inner part of the “caps” is also made of donnayite-(Y). The crystals are 1–3 mm in size. The yellow “caps” were used by Thi et al. (1992) to study the crystal structure of the trigonal polymorph of “donnayite-(Y)” (Igor Pekov, personal communication, 2021). That is likely why the proposed structure was characterised by the lack of Ca. The specimen is currently in Pekov's collection, no. 7660; the subsample that we studied was deposited at the Canadian Museum of Nature, specimen CMNMC 90476. This is the specimen which Alexander Khomyakov used for the first detailed description of donnayite-(Y) from the Khibiny Massif and that Galina Dorokhov used to draw donnayite-(Y) crystals on the basis of goniometric measurements. The drawings are featured in Khomyakov's famous monograph Mineralogy of Hyperagpaitic Alkaline Rocks (Khomyakov, 1995).
At all three localities alicewilsonite-(YCe) is a late-stage mineral found in peralkaline geological environments. Presumed alicewilsonite-(YCe) from the Narssârssuk pegmatite described by Petersen et al. (2003) UK-33A was formed in a similar environment.
Alicewilsonite-(YCe) varies in colour from pale yellow to yellow, lemon yellow, green yellow, orange-yellow, pale green to green, pale grey to grey, green grey, beige, and colourless (Figs. 1, 3). The streak is white; the lustre is vitreous. The cleavage is fair to indistinct, parallel to {001}. The fracture is uneven. The Mohs hardness is 3. The mineral is non-fluorescent under ultraviolet light. The density calculated using the empirical formula and unit cell volume refined from the single-crystal XRD data is 3.37 g cm−3.
Alicewilsonite-(YCe) is optically biaxial (+), with α=1.554(3), β=1.558(3), γ=1.644(2), 2V (calc.) = 26∘, 2V (meas.) = 20(3)∘ (from a spindle-stage extinction curve). Orientation is X∧c is ca. 5∘.
Electron microprobe analyses (EMPAs) for alicewilsonite-(YCe) were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five WDS spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) with an acceleration voltage of 20 kV, a beam current of 10 nA, and a beam diameter of 20–50 µm depending on the grain size. Alicewilsonite-(YCe) is unstable under an electron beam, and so a larger beam diameter was used to minimise element migration. The following reference materials were used: albite or NaInSi2O6 (NaKα), diopside (CaKα), celestine (SrLα), sanbornite (BaLα), YAG (YLα), LaPO4 (LaLα), CePO4 (CeLα), PrPO4 (PrLβ), NdPO4 (NdLα), SmPO4 (SmLα), EuPO4 (EuLα), GdPO4 (GdLα), TbPO4 (TbLα), DyPO4 (DyLβ), HoPO4 (HoLβ), ErPO4 (ErLα), YbPO4 (YbLα), zircon (ZrLα), and ThO2 (ThMα). The intensity data were corrected for time-dependent intensity (TDI) loss (or gain) using a self-calibrated correction for NaKα, CaKα, YLα, and LaLα. H2O and CO2 contents were not analysed due to the paucity of the available material. Despite the relatively large size of some of the crystals, they are zoned, and it was impossible to separate alicewilsonite-(YCe) as an individual phase in a large enough amount for the analysis.
The Fourier transform infrared (FTIR) spectrum of alicewilsonite-(YCe) was obtained using a Bruker Hyperion 2000 microscope interfaced to a Tensor 27 spectrometer with a wide-band mercury cadmium telluride (MCT) detector (Canadian Conservation Institute, Canada). A small fragment of alicewilsonite-(YCe) was mounted on a low-pressure diamond anvil microsample cell and analysed in transmission mode. The spectrum was collected between 4000–400 cm−1 with the co-addition of 150 scans at a 4 cm−1 resolution.
Powder X-ray diffraction data (PXRD) were collected at the Canadian Museum of Nature, Canada, using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and IμS microfocus X-ray source (λCuKα1 = 1.54060 Å) with the Kα2 contribution removed using the “Strip Kα2” tool in Bruker Diffrac.EVA V4.3. The instrument was calibrated using a statistical calibration method (Rowe, 2009). A powder ball 200 µm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10∘ rocking motion along the Psi axis of the centric Eulerian cradle stage.
Single-crystal X-ray diffraction (SXRD) studies were carried out using a Rigaku XtaLAB Synergy-S diffractometer equipped with a HyPix 6000HE detector (λMoKα = 0.71073 Å) operating at 50 kV and 1 mA at the Natural History Museum, University of Oslo, Norway. The data were processed, including absorption correction, using Rigaku's CrysAlis Pro software (Matsumoto et al., 2021). The studied grain was sampled from the rim a crystal from the holotype and was composed of only alicewilsonite-(YCe).
6.1 Chemical data
Representative EMPAs for alicewilsonite-(YCe) from Mont Saint-Hilaire and the Saint-Amable sill (both in Quebec, Canada) and the Khibiny Massif (Kola Peninsula, Russia) are given in Table 1. An analysis of the phase used for the structural study of “trigonal donnayite-(Y)” by Thi et al. (1992) is included (no. 7). The contents of U, Tm, Lu, and Hf are below the detection limit.
The data on the holotype of alicewilsonite-(YCe) were collected on a crystal later used for the crystal structure study. The empirical formula calculated on the basis of six cations, excluding H+, is Na2.11Ca0.11Sr1.83Ba0.08Y0.67(Ce0.51La0.32 Pr0.03Nd0.09Sm0.02Gd0.04Dy0.08Ho0.02Er0.06 Yb0.03)Σ1.20(CO3)5.88(H2O)3.00. The simplified formula is Na2(Sr,Ca)2(Y,Dy,Er,Na)(Ce,La,Nd)(CO3)6 ⋅ 3H2O.
The ideal end-member formula is Na2Sr2YCe(CO3)6 ⋅ 3H2O, which requires Na2O 7.17, SrO 23.98, Y2O3 13.06, Ce2O3 18.99, CO2 30.55, H2O 6.25, total 100 wt. %.
Alicewilsonite-(YCe) dissolves in an aqueous HCl solution at room temperature with strong effervescence.
6.2 Infrared spectroscopy
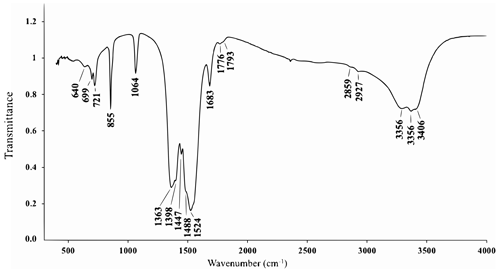
The IR spectrum of the holotype of alicewilsonite-(YCe) (Fig. 4) shows IR bands of O–H-stretching (in the range from 3350 to 3410 cm−1) and H–O–H bending (at 1683 cm−1) vibrations of H2O molecules and C–O-stretching (in the range 1350–1550 cm−1) vibrations of CO-group molecules. The band at 1064 cm−1 can be assigned to the non-degenerate mode of C–O stretching vibrations, indicating polarisation of CO groups, as this mode would be inactive, if symmetric non-polarised carbonate groups (with a three-fold axis) were present in the IR spectrum. The band assignment was made in accordance with Chukanov and Chervonnyi (2016).
Table 2X-ray powder diffraction data (d in Å) for alicewilsonite-(YCe). The strongest reflections are given in bold.
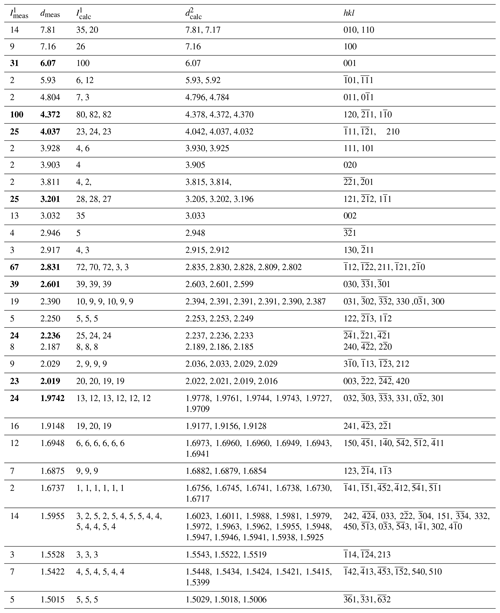
1 Calculated from the crystal structure determination, only reflections with intensities >1 are given. 2 Calculated from PXRD Rietveld unit cell refinement with a=9.0036(6), b=9.0175(6), c=6.7712(5) Å, α=102.724(2), β=116.398(2), γ=60.003(2), and V=426.46(5) Å3.
6.3 X-ray diffraction data and description of the crystal structure
The indexed PXRD data for the holotype of alicewilsonite-(YCe) are given in Table 2. Parameters of the triclinic unit cell refined from the data are as follows: a=9.0036(6) Å, b=9.0175(6) Å, c=6.7712(5) Å, α=102.724(2)∘, β=116.398(2)∘, γ=60.003(2)∘, and V=426.46(5) Å3. The PXRD pattern in the xy format is available in the Supplement.
Alicewilsonite-(YCe) studied by Thi et al. (1992) as “trigonal donnayite-(Y)” also turned out to be triclinic with the following unit cell parameters refined from PXRD data: a=8.9859(9) Å, b=9.0266(8) Å, c=6.7849(6) Å, α=102.555(8)∘, β=116.337(7)∘, γ=59.939(8)∘, and V=426.86(8) Å3.
Table 3Crystal data, data collection information, and structure refinement details for alicewilsonite-(YCe).
∗ Located 0.74 Å away from the Ce3 site. There are several 2.0–2.8 e/Å−3 peaks located 0.7–0.8 Å away from large cations sites that indicate a relatively poor overall quality of the data (caused by a poor quality of alicewilsonite-(YCe) crystals), which led to spurious peaks and holes of residual electron density.
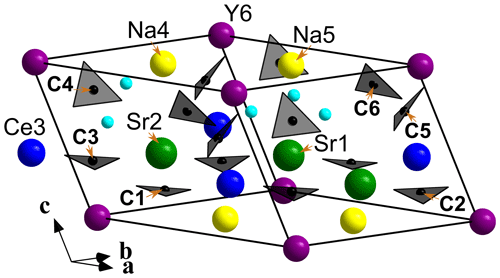
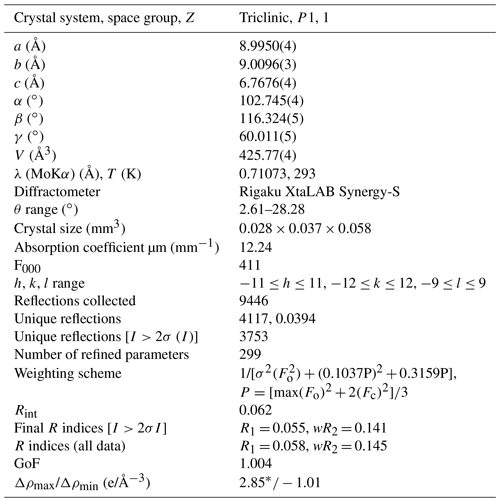
Figure 5General view of the crystal structure of alicewilsonite-(YCe). Sky-blue spheres are H2O molecules. The unit cell is outlined.
The SXRD data for the holotype of alicewilsonite-(YCe) were indexed in the P1 space group with the following unit cell parameters: a=8.9950(4) Å, b=9.0096(3) Å, c=6.7676(4) Å, α=102.745(4)∘, β=116.324(5)∘, γ=60.011(5)∘, and V=425.77(4) Å3. The structure was solved and refined to R1 = 0.055 on the basis of 3753 independent reflections with I>2σ(I) using the SHELXL-2018/3 program package (Sheldrick, 2015). Crystal data, data collection information, and structure refinement details are given in Table 3; atom coordinates, equivalent displacement parameters, site composition, and bond valence sums (BVS) in Table 4; and selected interatomic distances in Table 5. The studied crystal demonstrated twinning by merohedry Class I (Nespolo and Ferraris, 2000) – inversion twinning, with a twin domain ratio of 87:13. A crystallographic information file (CIF) for alicewilsonite-(YCe) is available as a Supplement. It was also deposited in the Inorganic Crystal Structure Database (ICSD; no. CSD 2223160).
Figure 6A view along [010] of the crystal structure of alicewilsonite-(YCe). Sr-, Ce-, Y-, and Na-centred polyhedra are shown in green, blue, purple, and yellow, respectively. Carbonate groups are black triangles. The unit cell is outlined.
Table 4Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms, site occupancies, and bond valence sums (BVSs) for alicewilsonite-(YCe).
∗ The site occupancies were refined assuming full occupancy. The best agreement was obtained with Sr0.728(12)Ce0.272(11) for the Sr1 site, Sr0.686(11)Ce0.314(11) for the Sr2 site, Ce0.598(10)Sr0.402(11) for the Ce3 site, Na0.956(5)Dy0.044(5) for the Na5 site, and Y0.929(8)Dy0.071(7) for the Y6 site. In the final refinement cycles the occupancies were fixed based on the eref values, EMPA, interatomic distances, bond valence calculations, and the charge balance. Bond valence parameters were taken from Brese and O'Keeffe (1991).
Single-crystal X-ray studies of the mckelveyite group minerals are very challenging because of the poor quality of their crystals resulting in multiple split reflections and streaks. Alicewilsonite-(YCe) is no exception, and because of the relatively low quality of our data (R1=0.055), we were unable to extract much detail about the structure. Nevertheless, the data allowed us to reliably distribute the dominant cations and present a simplified model of the crystal structure of the new mineral.
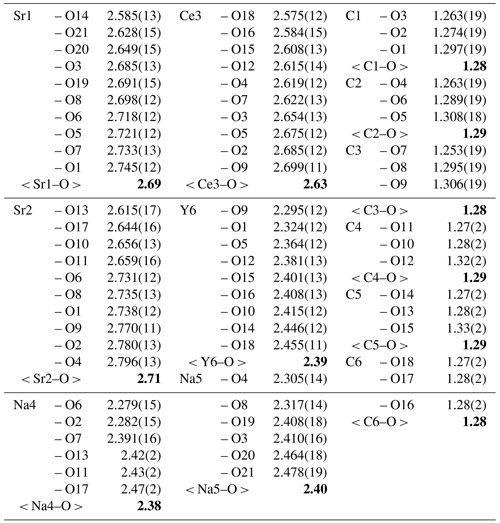
Alicewilsonite-(YCe) is strongly pseudotrigonal. There are six independent large cation sites in the structure (Fig. 5) forming two alternating layers parallel to the ab plane (Fig. 6). Na, Sr, Y, Ce, and Ba were distributed among these sites based on the EMPA, refined site-scattering factors (eref, in electrons per site), and charge balance taking into account bond valence sums (BVS) and interatomic distances (Tables 4–5). Ca atoms were not included in the refinement; lighter lanthanoids (Ln: La–Lu) were formally refined as Ce atoms, heavier Ln – as Dy atoms. One of the layers is formed by the Sr1, Sr2, and Ce3 sites that have a 10-fold coordination. The refinement showed that Sr and Ce are distributed between all three sites; the best agreement was obtained with the refined site-occupation factor Sr0.728(12)Ce0.272(11) for the Sr1 site, Sr0.686(11)Ce0.314(11) for the Sr2 site, and Ce0.598(10)Sr0.402(11) for the Ce3 site. Thus, Sr atoms occupy predominantly larger Sr1- and Sr2-centred polyhedra (the average interatomic distances are 2.69 Å for < Sr1–O > and 2.71 Å for < Sr2–O >), while Ce atoms prefer the smaller Ce3-centred polyhedron with the < Ce3–O > distance of 2.63 Å. The occupancies were assigned as Sr0.73Ce0.23Ba0.05Sr0.67Ce0.28Ba0.05, and Ce0.60Sr0.40 for the Sr1, Sr2, and Ce3 sites, respectively. Na4 and Na5 sites are six-coordinated sites. The Na4 site is occupied by Na atoms, while the refinement of the Na5 site occupancy [Na0.956(5)Dy0.044(5)] indicated an admixture of heavier cations at the site, so the occupancy was formally fixed as Na0.95Dy0.05. The Y6 site is occupied predominantly by Y atoms (80 %) with admixed Na (10 %) and Dy (10 %) atoms. The Y6-centred nine-fold polyhedron has the < Y6–O > distance of 2.39 Å, which is close to the average Y–O distance (2.41 Å) observed in mckelveyite-(Y)-2M (Demartin et al., 2008).
Table 5Selected interatomic distances (Å) in the structure of alicewilsonite-(YCe). The average interatomic distances are given in bold.
The arrangement of the six carbonate groups is similar to that of weloganite (Grice and Perrault, 1975). The three CO groups centred by carbon atoms C1, C2, and C3 are almost coplanar with {001}, while the other three centred by C4, C5, and C6 atoms are not coplanar (Fig. 5).
The BVS at the O19, O20, and O21 sites (0.41, 0.42, and 0.42 valence units, respectively) indicate the presence of H2O0 molecules, also confirmed by the presence of the bands of O–H-stretching and H–O–H bending vibrations in the IR spectrum of alicewilsonite-(YCe). The three H2O molecules are bonded to Sr1- and Na5-centred polyhedra that share a face.
The resulting structural formula of alicewilsonite-(YCe) is
which leads to the ideal formula Na2Sr2YCe(CO3)6 ⋅ 3H2O.
Table 6Comparative data for alicewilsonite-(YCe) and related mckelveyite group minerals.
∗Data for triclinic polytypes are given. The data for the original description (Chao et al., 1978) were collected on two species: alicewilsonite-(YCe) and donnayite-(Y) (Lykova et al., 2023); therefore, for this table we used the data collected during re-investigation of donnayite-(Y) type specimens.
The structural formula is in good agreement with the empirical formula
where LREE (light rare-earth elements) = Ce0.51La0.32Nd0.09Pr0.03Sm0.02, and HREE (heavy rare-earth elements) = Dy0.08Er0.06Gd0.04Ho0.02Yb0.03.
Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, is a member of the mckelveyite group (Lykova et al., 2023). It is the NaCe analogue of donnayite-(Y), NaCaSr3Y(CO3)6 ⋅ 3H2O (Chao et al., 1978); the entry of LREE follows the coupled substitution scheme Ca Sr LREE3+. It is also closely related to mckelveyite-(Y), NaCaBa3Y(CO3)6 ⋅ 3H2O (Milton et al., 1965; Chao et al., 1978; Demartin et al., 2008), and weloganite, Na2Sr3Zr(CO3)6 ⋅ 3H2O (Sabina et al., 1968; Grice and Perrault, 1975; Table 6).
At Mont Saint-Hilaire alicewilsonite-(YCe) and donnayite-(Y) usually occur as distinct phases, and when found in one crystal, the boundary between the phases is sharp. Phases with composition intermediate between alicewilsonite-(YCe) and donnayite-(Y) are rare, the content of Ca in alicewilsonite-(YCe) usually does not exceed 0.3 atoms per formula unit (apfu), and we have not found a crystal yet where the composition would change gradually from alicewilsonite-(YCe) to donnayite-(Y), or vice versa. In addition, there is no clear genetic trend as either mineral can be found forming an epitactic overgrowth on another; although, epitaxy of alicewilsonite-(YCe) on donnayite-(Y) is somewhat more common.
At the Khibiny Massif, on the other hand, phases with intermediate composition (0.3–0.7 apfu Ca) are common and the composition may vary gradually within one crystal. A typical example is given in Table 1 (analysis no. 7); more analyses were published by Pekov and Podlesnyi (2004). Moreover, in all specimens that we studied, alicewilsonite-(YCe) was always the later phase, either forming an epitactic overgrowth on donnayite-(Y) or even replacing the latter.
Some alicewilsonite-(YCe) specimens are characterised by a higher content of heavier Ln, especially Nd, Sm, and Dy, and a lower Y content (Table 1; analyses nos. 4 and 6), while the Ce and La contents remain constant, confirming the Y HREE3+ substitution scheme at the Y6 site and showing that Nd and Sm atoms can also selectively concentrate at that site. These phases appear to be more typical for the Khibiny Massif, which is consistent with the observation that alicewilsonite-(YCe) is usually the later mckelveyite group mineral at Khibiny and as such is formed from late highly differentiated solutions rich in heavier Ln.
Ordering of Y + Ln between different positions has been described in several minerals. In crichtonite group minerals, LREE and Y + HREE are located at different sites, as was shown on the structure of davidite-(La), La(U,Y)Fe2(Ti,Fe,Cr,V)18(O,OH)38, reconstituted by heating (Gatehouse et al., 1979). In tveitite-(Y), (Y,Na)6(Ca,LREE)6(Ca,Na,HREE)6(Ca,Na)F42, Y, LREE, and HREE are also ordered between different sites (Yakubovich et al., 2007). Alicewilsonite-(YCe) is another example of crystal–chemical-driven ordering of lighter and larger Ln at the larger cation sites and Y + heavier and smaller Ln at the smaller cation sites within one structure.
Zr is sometimes present as a small admixture in alicewilsonite-(Y) (Table 1; analysis no. 4); its entry in the structure follows the coupled substitution scheme Ca Y Na Zr4+ also realised in weloganite. Th is also sometimes present (Table 1; analyses nos. 2, 4 and 6). Its entry could follow a similar substitution mechanism (Ca Y Na Th4+); however, Th4+ cations are significantly larger than Zr4+ cations, and the degree of tolerance of the weloganite-type structures to Th cations is unclear.
Over the course of this study we have not found a monoclinic or trigonal phase with alicewilsonite-(YCe) chemistry. As shown above, the trigonal R3m polymorph of “donnayite-(Y)” with the structural formula (Na,REE)Sr(CO3)2 ⋅ H2O reported by Thi et al. (1992) was very likely triclinic alicewilsonite-(YCe). The strongly trigonal pseudosymmetry of the mckelveyite group minerals can make it difficult to discern their true symmetry, especially when using older diffractometers. In fact, both mckelveyite-(Y) and weloganite were originally reported to be trigonal (Milton et al., 1965; Sabina et al., 1968). On the other hand, the existence of a high-symmetry, more disordered, higher-temperature modification of alicewilsonite-(YCe) would not be at variance with what we currently know about mckelveyite group minerals. Further investigation is needed before a definite conclusion can be reached.
Crystallographic data for alicewilsonite-(YCe) and its PXRD pattern in the xy format are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-35-143-2023-supplement.
IL conceptualised the project. RR collected powder X-ray diffraction data and subsampled the specimens. EMPAs were obtained by GP. HF collected X-ray single-crystal diffraction data. KH obtained the IR spectrum. IL processed the data and interpreted the results. The manuscript was written by IL with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank Francesco Demartin and an anonymous referee for their valuable comments, Laszlo Horvath, Elsa Pfenninger-Horvath, Peter Tarassoff, and Igor Pekov for providing us with samples for the study and François Génier and Michael Bainbridge for taking the colour photos.
This research was financially supported by the Canadian Museum of Nature.
This paper was edited by Sergey Krivovichev and reviewed by Francesco Demartin and one anonymous referee.
Bayliss, P. and Levinson, A. A.: A system of nomenclature for rare-earth mineral species; revision and extension, Am. Mineral., 73, 422–423, 1988.
Brese, N. E. and O'Keeffe, M.: Bond-valence parameters for solids, Acta Crystallogr. B, 47, 192–197, https://doi.org/10.1107/S0108768190011041, 1991.
Chao, G. Y., Mainwaring, P. R., and Baker, J.: Donnayite, NaCaSr3Y(CO3)6 ⋅ 3H2O, a new mineral from Mont St-Hilaire, Quebec, Can. Mineral., 16, 335–340, 1978.
Chao, G. Y., Conlon, R. P., and Van Velthuizen, J.: Mont Saint-Hilaire unknowns, Mineral. Rec., 21, 363–368, 1990.
Chukanov, N. V. and Chervonnyi, A. D.: Infrared Spectroscopy of Minerals and Related Compounds, Springer Cham, Switzerland, 1109 pp., https://doi.org/10.1007/978-3-319-25349-7, 2016.
Donnay, G., Donnay, J. D. H., and Hey, M. H.: Ewaldite, a new barium calcium carbonate, I. Occurrence of ewaldite in syntactic intergrowth with mackelvyite, Tscher. Miner. Petrog., 15, 185–200, https://doi.org/10.1007/BF01087021, 1971.
Demartin, F., Gramaccioli, C. M., Campostrini, I., and Diella, V.: The crystal structure of mckelveyite-(Y)-2M, a new monoclinic polytype from Val Malenco, Italian Alps, Can. Mineral., 46, 195–203, https://doi.org/10.3749/canmin.46.1.195, 2008.
Gatehouse, B. M., Grey, I. E., and Kelly, P. R.: The crystal structure of davidite, Am. Mineral., 64, 1010–1017, 1979.
Grice, J. D. and Perrault, G.: The crystal structure of triclinic weloganite, Can. Mineral.,, 13, 209–216, 1975.
Horvath, L., Pfenninger-Horvath, E., Gault, R. A., and Tarassoff, P.: Mineralogy of the Saint-Amable Sill, Varennes and Saint-Amable, Quebec, Mineral. Rec., 29, 83–118, 1998.
Horvath, L., Gault, R. A., Pfenninger-Horvath, E., and Poirier, G.: Mont Saint-Hilaire: History, Geology, Mineralogy, The Canadian Mineralogist Special Publication 14, Mineralogical Association of Canada, Canada, 634 pp. 2019.
Khomyakov, A. P.: Mineralogy of Hyperagpaitic Alkaline Rocks., Clarendon Press, Oxford, 223 pp.,1995.
Levinson, A. A.: A system of nomenclature for rare-earth minerals, Am. Mineral., 51, 152–158, 1966.
Lykova, I., Rowe, R., Poirier, G., Giester, G., Ojaste, K., and Friis, H.: Mckelveyite group minerals – Part 1: Nomenclature and new data on donnayite-(Y), Eur. J. Mineral., 35, 133–142, https://doi.org/10.5194/ejm-35-133-2023, 2023.
Matsumoto, T., Yamano, A., Sato, T., Ferrara, J. D., White, F. J., and Meyer, M.: “What is This?” A structure analysis tool for rapid and automated solution of small molecule structures, J. Chem. Crystall., 51, 438–450, 10.1007/s10870-020-00867-w, 2021.
Milton, C., Ingram, B., Clark, J. R., and Dwornik, E. J.: Mckelveyite, a new hydrous sodium barium rare-earth uranium carbonate mineral from The Green River Formation, Wyoming, Am. Mineral., 50, 593–612, 1965.
Nespolo, M. and Ferraris, G.: Twinning by syngonic and metric merohedry. Analysis, classification and effects on the diffraction pattern, Z. Krist.-Cryst. Mater., 215, 77–81, https://doi.org/10.1524/zkri.2000.215.2.77, 2000.
Pekov, I. V. and Podlesnyi, A. S.: Kukisvumchorr Deposit: Mineralogy of Alkaline Pegmatites and Hydrothermalites, Mineralogical Almanac, Ekost association, Russia, 176 pp. 2004.
Petersen, O. V., Balic-Zunic, T., Gault, R. A., and Andersen, T.: The first occurrence of ewaldite and donnayite-(Y), two rare carbonate minerals, in the Narssârssuk pegmatite, South Greenland, Neues Jahrbuch für Mineralogie – Monatshefte, 2003, 543–555, https://doi.org/10.1127/0028-3649/2003/2003-0543, 2003.
Sabina, A. P., Jambor, J. L., and Plant, A. G.: Weloganite, a new strontium zirconium carbonate from Montreal Island, Canada, Can. Mineral., 9, 468–477, 1968.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr., 71, 3–8, https://doi.org/10.1107/s2053229614024218, 2015.
Rowe, R.: New statistical calibration approach for Bruker AXS D8 Discover microdiffractometer with Hi-Star detector using GADDS software, Powder Diffraction, 24, 263–271, https://doi.org/10.1154/1.3193683, 2009.
Thi, T. T. L., Pobedimskaja, E. A., Nadezhina, T. N., and Khomyakov, A. P.: Polymorphism of donnayite, (Na,TR)Sr(CO3)2 ⋅ H2O, Vestnik Moskovskogo Universiteta, Geologiya, 47, 60–70, 1992.
Thi, T. T. L., Pobedimskaja, E. A., Nadezhina, T. N., and Khomyakov, A. P.: The crystal structures of alkaline carbonates: barentsite, bonshtedtite and donnayite, Acta Crystallogr. A, 40, C257, https://doi.org/10.1107/S0108767384092291, 1984.
Voloshin, A. V., Subbotin, V. V., Yakovenchuk, V. N., Pakhomovskiy, Y. A., Men'shikov, Y. P., Nadezhdina, T. N., and Pushcharovsky, D. Y.: New data on ewaldite, Zapiski Vserossijskogo Mineralogicheskogo Obshchestva, 121, 56–67, 1992.
Yakubovich, O. V., Massa, W., Pekov, I. V., and Gavrilenko, P. G.: Crystal structure of tveitite-(Y): Fractionation of rare-earth elements between positions and the variety of defects, Crystallogr. Rep., 52, 71–79, https://doi.org/10.1134/S1063774507010087, 2007.
- Article
(11522 KB) - Full-text XML