the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Mckelveyite group minerals – Part 1: Nomenclature and new data on donnayite-(Y)
Inna Lykova
Ralph Rowe
Glenn Poirier
Gerald Giester
Kelsie Ojaste
Henrik Friis
The mckelveyite group consisting of seven carbonate minerals – mckelveyite-(Y), ewaldite, weloganite, donnayite-(Y), alicewilsonite-(YCe), alicewilsonite-(YLa), and bainbridgeite-(YCe) – is formally established. The general formula of the minerals is A3B3(CO3)6 ⋅ 3H2O, where A= Na, Ca, Y, and Zr and B= Sr, Ba, Ce, and La. Different order–disorder modifications are known resulting in triclinic, monoclinic, hexagonal, and trigonal minerals with essentially the same structure. Re-examination of donnayite-(Y) type specimens shows that the original description contains data collected on two different species: donnayite-(Y) and alicewilsonite-(YCe). Donnayite-(Y), NaCaSr3Y(CO3)6 ⋅ 3H2O, was found in only one specimen out of seven – CMNMC 39396 – housed at the Canadian Museum of Nature, Ottawa. This specimen becomes the holotype of donnayite-(Y). The crystal structure of donnayite-(Y) was solved and refined to R1= 0.055 for 3366 reflections with I>2σ(I). Donnayite-(Y) is shown to have a weloganite-type structure confirming its place in the mckelveyite group.
- Article
(3855 KB) - Full-text XML
- Companion paper 2
- Companion paper 3
- Companion paper 4
-
Supplement
(275 KB) - BibTeX
- EndNote
The terms “donnayite group” (Demartin et al., 2008; Horvath et al., 2019) and “mckelveyite group” (Frost et al., 2013) can be found in the literature describing four related carbonate minerals donnayite-(Y) (Chao et al., 1978), mckelveyite-(Y) (Milton et al., 1965), ewaldite (Donnay et al., 1971), and weloganite (Sabina et al., 1968), but neither the group nor its name had previously been approved by Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA CNMNC).
We conducted a comprehensive investigation of these minerals that resulted in the discovery of three new minerals species – alicewilsonite-(YCe) (IMA 2020-055; Lykova et al., 2023), bainbridgeite-(YCe) (IMA 2020-065), and alicewilsonite-(YLa) (IMA 2021-047) – the holotype redefinition for donnayite-(Y) (proposal 22-F), and the formal establishment of the mckelveyite group (proposal 22-G), all approved by the CNMNC.
This paper, the first one in the series on the mckelveyite group minerals, presents the nomenclature of the mckelveyite group and new data on donnayite-(Y), including first known data on its crystal structure.
Mckelveyite-(Y), a Na–Ca–Ba–Y carbonate, from the Green River formation, Wyoming, USA, was the first mineral in the group to be described (Milton et al., 1965). Subsequently weloganite from the Francon quarry, Quebec, Canada (Sabina et al., 1968), and ewaldite also from the Green River formation (Donnay et al., 1971) were described.
Solving crystal structures of mckelveyite group minerals proved to be challenging due to the high degree of disorder. The ewaldite structure was determined by Donnay and Preston (1971), and the formula Ba(Ca,REE,Na,K,Sr,U)(CO3)2 (Z = 2) was suggested. The new idealised formula BaCa(CO3)2 ⋅ 2.6H2O (Z = 2; P63mc) was proposed by Voloshin et al. (1992), who studied material from the Vouriyarvi Massif, Russia. It was suggested that mckelveyite-(Y) and ewaldite may be polymorphs. Both studies provided only limited structural characterisation due to the challenging nature of the material. Weloganite was the first triclinic group member with a determined structure (Chen and Chao, 1975; Grice and Perrault, 1975). The proposed formula was Na2Sr3Zr(CO3)6 ⋅ 3H2O (Z = 1).
Three years later donnayite-(Y), NaCaSr3Y(CO3)6 ⋅ 3H2O, was described from Mont Saint-Hilaire, Quebec, Canada (Chao et al., 1978). The authors concluded that it is the Ca–Y analogue of weloganite and the Sr analogue of mckelveyite-(Y) and proposed the mckelveyite-(Y) end-member formula: NaCaBa3Y(CO3)6 ⋅ 3H2O. Chao et al. (1978) did not provide structural data on donnayite-(Y). Thi et al. (1984, 1992) reported crystal structures of two “donnayite-(Y)” polymorphs – triclinic P1 and trigonal R3m on material from the Khibiny Massif, Kola Peninsula, Russia. Both phases were characterised by (1) the lack of Ca and (2) the Na : REE ratio close to 1:1, indicating that the crystals were a different species. We are unaware of any published structural work on actual donnayite-(Y).
Demartin et al. (2008) proposed the first model of the crystal structure of mckelveyite-(Y) confirming the NaCaBa3Y(CO3)6 ⋅ 3H2O (Z = 4) formula. It is the only known structural study of a monoclinic Cc polymorph with the weloganite-type structure.
Three new minerals with weloganite-type structures (P1) were recently approved by the IMA: alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O (IMA 2020-055; Lykova et al., 2023), and bainbridgeite-(YCe), Na2Ba2YCe(CO3)6 ⋅ 3H2O (IMA 2020-065), from Mont Saint-Hilaire, and alicewilsonite-(YLa), Na2Sr2YLa(CO3)6 ⋅ 3H2O (IMA 2021-047), from the Paratoo Mine, Australia. It is likely that two “donnayite-(Y)” polymorphs reported by Thi et al. (1984, 1992) were, in fact, alicewilsonite-(YCe), which corroborates with the EMPA we obtained on one of the specimens studied by Thi et al. (Lykova el al, 2023).
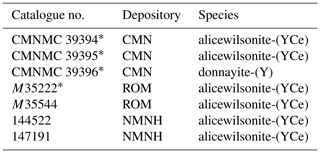
Donnayite-(Y), NaCaSr3Y(CO3)6 ⋅ 3H2O, was described by Chao et al. (1978) in several specimens from Mont Saint-Hilaire, Quebec, Canada. According to the original publication there were four cotype specimens deposited at the National Museum of Natural Sciences (now – Canadian Museum of Nature), Ottawa (CMN; specimens CMNMC 39394, CMNMC 39395, and CMNMC 39396), and the Royal Ontario Museum, Toronto (ROM; specimen M35222). Three other specimens not mentioned in the article were catalogued as cotypes: two at the Smithsonian National Museum of Natural History, Washington, DC (NMNH; specimens 144 522 and 147 191), and one more specimen at the ROM (M35544).
Re-examination of donnayite-(Y) type specimens showed that the original description contains data collected on two different phases with simplified formulae NaCaSr3Y(CO3)6 ⋅ 3H2O and Na2Sr2YCe(CO3)6 ⋅ 3H2O (Table 1). The latter corresponds to the recently approved species alicewilsonite-(YCe) (Lykova et al., 2023). As the original formula of donnayite-(Y) was given as NaCaSr3Y(CO3)6 ⋅ 3H2O, and the mineral with that chemical composition has been consistently labelled as such from various localities around the world, we proposed to keep the name donnayite-(Y) for that phase.
Table 1Results of re-investigation of donnayite-(Y) cotypes.
* Specimens designated as cotypes in the original publication by Chao et al. (1978).
Donnayite-(Y) was found in only one specimen out of seven – CMNMC 39396 – housed at the Canadian Museum of Nature, Ottawa. This specimen becomes the holotype of donnayite-(Y). Results of its investigation are given below.
On the holotype specimen donnayite-(Y) forms transparent colourless hemimorphic barrel-shaped crystals less than 0.3 mm in size (Fig. 1).

Figure 1Colourless transparent donnayite-(Y) crystal, 0.2 mm in size. Specimen CMNMC 39396. Photo: François Génier.
3.1 Chemical data
Electron microprobe analyses (EMPAs) for donnayite-(Y) were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five WDS spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) with an acceleration voltage of 20 kV and a beam current of 10 nA. Donnayite-(Y) is unstable under an electron beam, and so a larger beam diameter of 30 µm was used to minimise element migration. The following reference materials were used: albite or NaInSi2O6 (NaKα), diopside (CaKα), celestine (SrLα), sanbornite (BaLα), YAG (YLα), LaPO4 (LaLα), CePO4 (CeLα), PrPO4 (PrLβ), NdPO4 (NdLα), SmPO4 (SmLα), EuPO4 (EuLα), GdPO4 (GdLα), TbPO4 (TbLα), DyPO4 (DyLβ), HoPO4 (HoLβ), ErPO4 (ErLα), and YbPO4 (YbLα). The intensity data were corrected for time-dependent intensity (TDI) loss (or gain) using a self-calibrated correction for NaKα, CaKα, YLα, and LaLα. H2O and CO2 contents were not analysed due to the paucity of the available material.
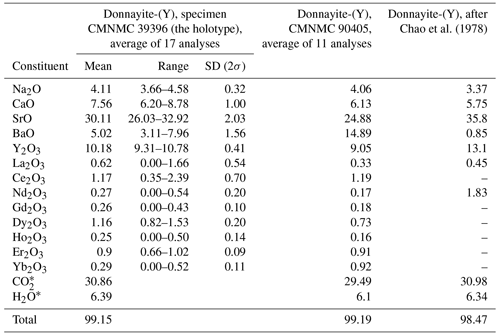
Chemical data are given in Table 2. The contents of U, Tm, Lu, Hf, and Th are below the detection limit. The empirical formula calculated on the basis of six cations, excluding H+, is Na1.13Ca1.14Sr2.46Ba0.28Y0.76La0.03Ce0.06Nd0.02Gd0.01 Dy0.05Ho0.01Er0.04Yb0.01(CO3)5.93(H2O)3.00. The simplified formula is NaCaSr3Y(CO3)6 ⋅ 3H2O.
Table 2Chemical data (in wt %) for donnayite-(Y) in comparison with data from Chao et al. (1978).
* Calculated from the stoichiometry.
Chao et al. (1978) analysed a number of specimens, but all except one were deemed unsuitable “owing to the presence of intimate syntactic intergrowths with other minerals”, which is how analyses corresponding to alicewilsonite-(YCe) were likely interpreted. Therefore, EMPAs were collected on one unspecified crystal only. Donnayite-(Y) from the original description is characterised by a higher content of Sr and lower Ba and Ca, which shows that the analysis was obtained on a different specimen that was not designated as a cotype. Summarising the history behind donnayite-(Y) discovery, Horvath et al. (2019) indicated that due to the nature of the material and its paucity, the species description required the use of several specimens collected, mostly before 1973, by different collectors including Peter Tarassoff, Elsa Pfenninger-Horvath, Laszlo Horvath, Marcelle Weber, William Henderson, and Quintin Wight. This corroborates our data and indicates that original EMPAs were likely collected on a specimen that is no longer identifiable.
3.2 Optical properties
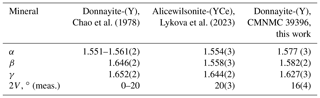
According to Chao et al. (1978) donnayite-(Y) is biaxial negative; however, we measured optical properties of several mckelveyite group minerals, and they all are biaxial positive, including alicewilsonite-(YCe) and donnayite-(Y). Comparative data given in Table 3 show that the original data were likely obtained on alicewilsonite-(YCe).
3.3 Powder X-ray diffraction data
Powder X-ray diffraction (PXRD) data on the holotype were collected at the Canadian Museum of Nature, Canada, using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and I µS microfocus X-ray source (λCuKα1= 1.54060 Å) with the Kα2 contribution removed using the “Strip Kα2” tool in Bruker Diffrac.EVA V4.3. The instrument was calibrated using a statistical calibration method (Rowe, 2009). A powder ball 200 µm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10∘ rocking motion along the Psi axis of the centric Eulerian cradle stage.
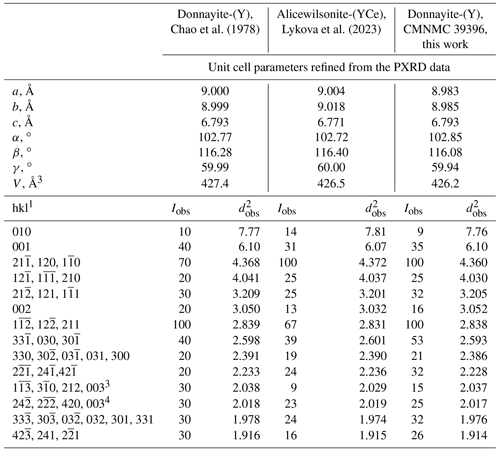
Parameters of the triclinic unit cell refined from the PXRD data are as follows: a= 8.9826(5) Å, b= 8.9853(5) Å, c= 6.7932(4) Å, α= 102.852(5)∘, β= 116.083(6)∘, γ= 59.935(4)∘, and V= 426.20(5) Å3. The PXRD pattern in the xy format is available as a Supplement file.
Comparative data for donnayite-(Y) and alicewilsonite-(YCe) are given in Table 4. The patterns are very similar. The unit cell parameter c of the alicewilsonite-(YCe) unit cell is somewhat smaller than that of donnayite-(Y), and the difference is notable in the basal reflections; however, both minerals are characterised by significant isomorphic substitutions at all cation sites, and thus varying unit cell parameters. The chemistry of the crystal used to collect PXRD data for the original study is unknown, so we cannot confidently tell if it was donnayite-(Y) or alicewilsonite-(YCe).
3.4 Single-crystal X-ray diffraction data and crystal structure of donnayite-(Y)
Single-crystal X-ray diffraction (SXRD) data for the holotype specimen CMNMC 39396 were (Fig. 1) collected at room temperature on a Rigaku XtaLAB Synergy-S diffractometer equipped with a HyPix 6000HE detector (λMoKα= 0.71073 Å) at the Natural History Museum, University of Oslo, Norway. The data were processed, including face indexed absorption correction, using Rigaku's CrysAlis Pro software (Matsumoto et al., 2021).
The data were indexed in the P1 space group with the following unit cell parameters: a= 8.9908(11) Å, b= 9.0067(11) Å, c= 6.8027(8) Å, α= 102.768(10)∘, β= 116.143(13)∘, γ= 60.000(13)∘, and V= 428.26(11) Å3.
Multiple domains, strong splitting and complex twinning were observed in every data set we collected. Therefore, we were only able to refine the structure to R1= 0.139 using the SHELXL 2018/3 program package (Sheldrick, 2015), and thus could not obtain a model of a reasonable quality.
We tested several crystals from different donnayite-(Y) specimens from Mont Saint-Hilaire, and specimen CMNMC 90405 from the Canadian Museum of Nature collection was deemed suitable for a single-crystal X-ray study. The studied donnayite-(Y) occurs as translucent green hemimorphic crystals up to 3 mm in size in a miarolitic cavity (Fig. 2). Chemical data are given in Table 2. The empirical formula calculated on the basis of 6 cations, excluding H+, is Na1.15Ca0.96Sr2.11Ba0.85Y0.70La0.02Ce0.06Nd0.01Gd0.01 Dy0.04Ho0.01Er0.04Yb0.04(CO3)5.89(H2O)3.00.

Figure 2Green hemimorphic donnayite-(Y) crystal. FOV 9 mm. Specimen CMNMC 90405. Photo: François Génier.
The SXRD data on specimen CMNMC 90405 were collected using a Bruker Kappa APEX II diffractometer equipped with a CCD detector and with an Incoatec Microfocus Source I µS (30 W, multilayer mirror, λMoKα) at the Institute of Mineralogy and Crystallography, University of Vienna, Austria.
The data were indexed in the P1 space group with the following unit cell parameters: a= 9.0273(6) Å, b= 9.0317(7) Å, c= 6.8517(7) Å, α= 102.600(3)∘, β= 115.990(4)∘, γ= 60.000(3)∘, and V= 434.86(7) Å3. The structure was solved and refined to R1= 0.055 on the basis of 3366 independent reflections with I>2σ(I) using the SHELXT and SHELXL-2018/3 programs (Sheldrick, 2015). Crystal data, data collection, and structure refinement details are given in Table 5; atom coordinates, equivalent displacement parameters, site composition, and bond valence sums (BVSs) in Table 6; selected interatomic distances in Table 7. The full bond valence calculations table was uploaded as a Supplement (Table S1).
Table 5Crystal data, data collection information, and structure refinement details for donnayite-(Y).
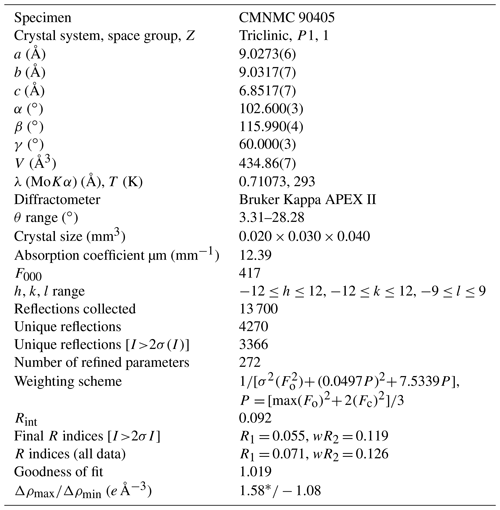
* Located 1.05 Å away from the O13 site.
A crystallographic information file (CIF) for donnayite-(Y) is available as a Supplement file. It was also deposited in the Inorganic Crystal Structure Database (ICSD; no. CSD 2223388).
Donnayite-(Y) is strongly pseudotrigonal. There are six independent large cation sites in the structure (Fig. 3) forming two alternating layers parallel to the ab plane (Fig. 4). Na, Ca, Sr, Ba, Y, and Dy were distributed among these sites based on the EMPA, refined site-scattering factors, and charge balance taking into account bond valence sums (BVSs) and interatomic distances (Tables 6–7). Ln (lanthanoids: La–Lu) were formally refined as Dy atoms. One of the layers is formed by the Sr1, Sr2, and Sr3 sites that have 10-fold coordination. The refinement showed that Sr and Ba are distributed between all three sites with the occupancies fixed as Sr0.70Ba0.30. Na4 and Ca5 sites have octahedral coordination, their occupancies were fixed as Na0.65Ca0.30Dy0.05 and Ca0.70Na0.27Dy0.03, respectively. The Y6 site is occupied predominantly by Y atoms (75 %) with admixed Dy (17 %) and Na (8 %) atoms.
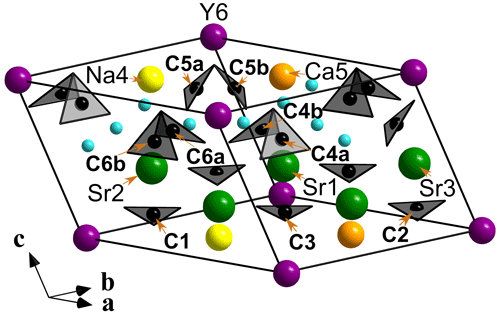
Figure 3General view of the crystal structure of donnayite-(Y). Sky-blue spheres are H2O molecules. The unit cell is outlined.
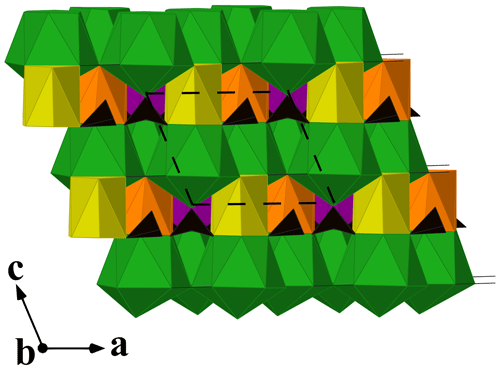
Figure 4A view along [010] of the crystal structure of donnayite-(Y). Sr-, Na-, Ca-, and Y-centred polyhedra are shown in green, yellow, orange, and purple, respectively. Carbonate groups are black triangles. The unit cell is outlined.
The three CO groups centred by carbon atoms C1, C2, and C3 are almost coplanar with {001} as in weloganite, Na2Sr3Zr(CO3)6 ⋅ 3H2O (Grice and Perrault, 1975). In the latter there are three other, non-coplanar CO groups centred by carbon atoms C4, C5, and C6. In donnayite-(Y) these three sites are split into two alternating sites each marked by letters a and b, e.g. C4a and C4b, forming alternating CO groups with a shared vertex (Fig. 3). The occupancy of the alternating carbon and oxygen sites was fixed at 50 %. Similar splitting was observed in the recently approved alicewilsonite-(YLa) (IMA 2021-047).
Table 6Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms, site occupancies, and bond valence sums (BVSs) for donnayite-(Y).
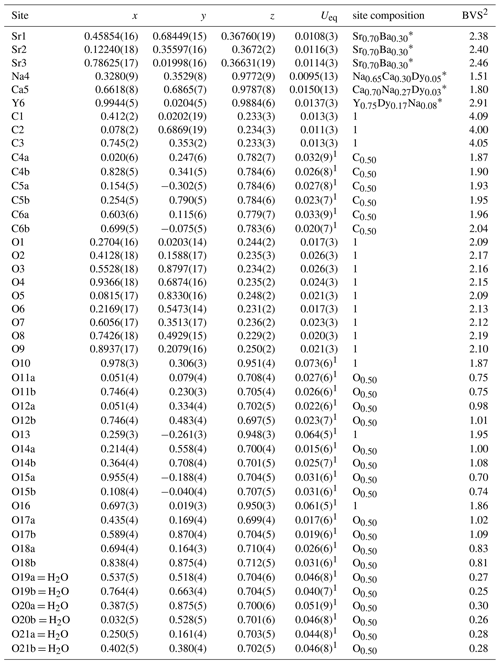
1 Uiso. 2 Bond valence parameters were taken from Brese and O'Keeffe (1991). * The sites occupancies were refined assuming full occupancy, and the best agreement was obtained with Sr0.648(15)Ba0.352(15) for the Sr1 site, Sr0.633(15)Ba0.367(15) for the Sr2 site, Sr0.704(16)Ba0.296(16) for the Sr3 site, Na0.863(8)Dy0.137(8) for the Na4 site, Ca0.906(10)Dy0.094(10) for the Ca5 site, and Y0.986(7) for the Y6 site. In the final refinement cycles the occupancies were fixed based on the site-scattering factors, EMPAs, interatomic distances, bond valence calculations, and the charge-balance.
Table 7Selected interatomic distances (Å) in the structure of donnayite-(Y).
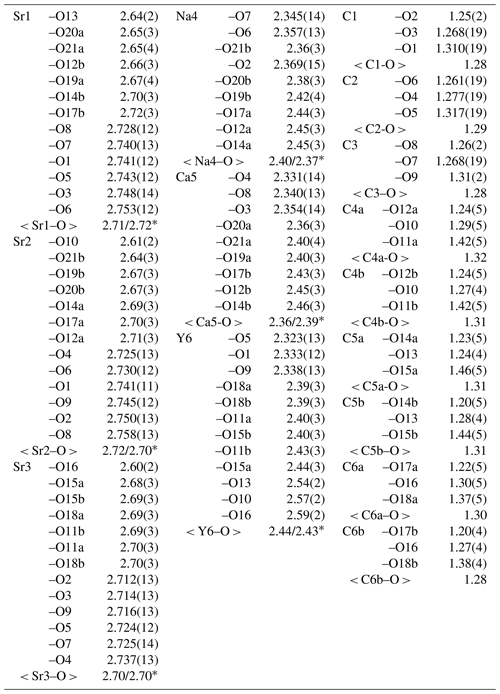
* Calculated for polyhedra with bonds M–O sites marked with appended “a”/M–O sites marked with appended “b”.
Table 8Mckelveyite group minerals.
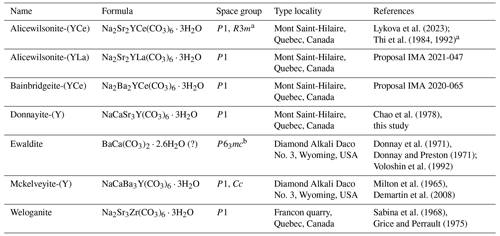
a The trigonal R3m polymorph of “donnayite-(Y)” reported by Thi et al. (1992) was very likely triclinic alicewilsonite-(YCe) (Lykova et al., 2023). b The studied structures were highly disordered; re-investigation is required to confirm the space group.
The BVSs at the O19a, O19b, O20a, O20b, O21a, and O21b sites (0.27, 0.25, 0.30, 0.26, 0.28, and 0.28 valence units, respectively) indicate the presence of H2O0 molecules. O19a, O20a, and O21a water molecules are bonded to Sr1- and Ca5-centred polyhedra that share a face, while O19b, O20b, and O21b water molecules are bonded to Sr2- and Na4-centred polyhedra.
Only one group of alternating C and O atoms marked by either “a” or “b” is occupied at the same time. Due to the quality of the collected data these atoms were not very reliably localised and were refined with isotropic atomic displacement parameters only.
The resulting structural formula of donnayite-(Y) is (Na0.65Ca0.30Dy0.05)∑1.00(Ca0.70Na0.27Dy0.03)∑1.00 (Sr2.10Ba0.90)∑3.00(Y0.75Dy0.17Na0.08)∑1.00(CO3)6(H2O)3, which leads to the ideal formula NaCaSr3Y(CO3)6 ⋅ 3H2O.
According to Mills et al. (2009) “a mineral group consists of two or more minerals with the same or essentially the same structure and composed of chemically similar elements”. Seven minerals – alicewilsonite-(YCe), alicewilsonite-(YLa), bainbridgeite-(YCe), donnayite-(Y), ewaldite, mckelveyite-(Y), and weloganite – meet the requirements for formation of a mineral group (Table 8).
Mckelveyite group minerals are carbonates with the general formula A3B3(CO3)6 ⋅ 3H2O, where A= Na, Ca, Y, Zr, and B= Sr, Ba, Ce, and La. Their structure is based on two alternating layers with larger cations B forming one layer and smaller cations A another. One half of the carbonate groups are coplanar to {001}, whereas another half is non-coplanar. Different order–disorder modifications occur resulting in triclinic, monoclinic trigonal, and hexagonal minerals with essentially the same structure (Donnay and Preston, 1971; Chen and Chao, 1975; Grice and Perrault, 1975; Thi et al., 1984, 1992; Voloshin et al., 1992; Demartin et al., 2008, our data). There are six independent cation sites in the structure of triclinic and monoclinic members and two sites in the trigonal and hexagonal members.
Although it is suggested “that the group name be that of the first mineral to have been adequately characterised” (Mills et al., 2009), a historical name was proposed for this group after the first mineral discovered in it – mckelveyite-(Y) (Milton et al., 1965). The historical significance should take precedence over the requirement of full structural study for an “adequate” characterisation of a species for this group due to challenges with structural determination because of strong disorder, multiple domains, splitting, and complex twinning. Moreover, although the first structural characterisation of a mckelveyite group mineral should be attributed to ewaldite (Donnay and Preston, 1971), many questions remain about its structure.
Crystal–chemical-driven ordering of lighter and larger Ln at the larger B sites and Y + heavier and smaller Ln at the smaller A sites is observed in the crystal structure of mckelveyite group minerals. In some cases, it leads to two different rare-earth elements being dominant at two different crystal-structure sites (alicewilsonite-(YCe), alicewilsonite-(YLa) and bainbridgeite-(YCe)). In those cases, two chemical symbols are appended to the name in accordance with the nomenclature for rare-earth and Y-mineral species (Levinson, 1966; Bayliss and Levinson, 1988). For the mckelveyite group minerals the first symbol represents the dominant cations at one of the A sites and the second symbol – at one of the B sites.
Crystallographic data for donnayite-(Y) and its PXRD pattern in the xy format are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-35-133-2023-supplement.
IL conceptualised the project. RR collected powder X-ray diffraction data and subsampled the specimens. EMPAs were obtained by GP. GG and HF collected single-crystal X-ray diffraction data. KO performed the observations of optical properties. IL processed the data and interpreted the results. The manuscript was written by IL with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We would like to thank Francesco Demartin and an anonymous referee for their valuable comments, the Royal Ontario Museum and Smithsonian National Museum of Natural History for loaning us the cotype specimens for this study, and François Génier for taking the colour photos.
This research has been supported by the Canadian Museum of Nature. Kelsie Ojaste's work was supported by a generous donation from Don Bubar.
This paper was edited by Sergey Krivovichev and reviewed by Francesco Demartin and one anonymous referee.
Bayliss, P. and Levinson, A. A.: A system of nomenclature for rare-earth mineral species; revision and extension, Am. Mineral., 73, 422–423, 1988.
Brese, N. E. and O'Keeffe, M.: Bond-valence parameters for solids, Acta Crystallogr. B, 47, 192–197, https://doi.org/10.1107/S0108768190011041, 1991.
Chao, G. Y., Mainwaring, P. R., and Baker, J.: Donnayite, NaCaSr3Y(CO3)6 ⋅ 3H2O, a new mineral from Mont St-Hilaire, Quebec, Can. Mineral., 16, 335–340, 1978.
Chen, T. T. and Chao, G. Y.: X-ray crystallography of weloganite, Can. Mineral., 13, 22–26, 1975.
Demartin, F., Gramaccioli, C. M., Campostrini, I., and Diella, V.: The crystal structure of mckelveyite-(Y)-2M, a new monoclinic polytype from Val Malenco, Italian Alps, Can. Mineral., 46, 195–203, https://doi.org/10.3749/canmin.46.1.195, 2008.
Donnay, G. and Preston, H.: Ewaldite, a new barium calcium carbonate, II. Its crystal structure, Tscher. Miner. Petrog., 15, 201–212, https://doi.org/10.1007/BF01087022, 1971.
Donnay, G., Donnay, J. D. H., and Hey, M. H.: Ewaldite, a new barium calcium carbonate, I. Occurrence of ewaldite in syntactic intergrowth with mackelvyite, Tscher. Miner. Petrog., 15, 185–200, https://doi.org/10.1007/BF01087021, 1971.
Frost, R. L., Xi, Y., Scholz, R., Belotti, F. M., and Filho, M. C.: Infrared and Raman spectroscopic characterization of the carbonate mineral weloganite – Sr3Na2Zr(CO3)6 ⋅ 3H2O and in comparison with selected carbonates, J. Mol. Struct., 1039, 101–106, https://doi.org/10.1016/j.molstruc.2013.02.005, 2013.
Grice, J. D. and Perrault, G.: The crystal structure of triclinic weloganite, Can. Mineral., 13, 209–216, 1975.
Horvath, L., Gault, R. A., Pfenninger-Horvath, E., and Poirier, G.: Mont Saint-Hilaire: History, Geology, Mineralogy, The Canadian Mineralogist Special Publication 14, Mineralogical Association of Canada, Canada, 634 pp., ISBN: 978-0-921294-61-0, 2019.
Levinson, A. A.: A system of nomenclature for rare-earth minerals, Am. Mineral., 51, 152–158, 1966.
Lykova, I., Rowe, R., Poirier, G., Friis, H., and Helwig, K.: Mckelveyite group minerals – Part 2: Alicewilsonite-(YCe), Na2Sr2YCe(CO3)6 ⋅ 3H2O, a new species, Eur. J. Mineral., 35, 143–155, https://doi.org/10.5194/ejm-35-143-2023, 2023.
Matsumoto, T., Yamano, A., Sato, T., Ferrara, J. D., White, F. J., and Meyer, M.: “What is This?” A structure analysis tool for rapid and automated solution of small molecule structures, J. Chem. Crystallogr., 51, 438–450, https://doi.org/10.1007/s10870-020-00867-w, 2021.
Mills, S. J., Hatert, F., Nickel, E. H., and Ferraris, G.: The standardisation of mineral group hierarchies: application to recent nomenclature proposals, Eur. J. Mineral., 21, 1073–1080, https://doi.org/10.1127/0935-1221/2009/0021-1994, 2009.
Milton, C., Ingram, B., Clark, J. R., and Dwornik, E. J.: Mckelveyite, a new hydrous sodium barium rare-earth uranium carbonate mineral from The Green River Formation, Wyoming, Am. Mineral., 50, 593–612, 1965.
Rowe, R.: New statistical calibration approach for Bruker AXS D8 Discover microdiffractometer with Hi-Star detector using GADDS software, Powder Diffr., 24, 263–271, https://doi.org/10.1154/1.3193683, 2009.
Sabina, A. P., Jambor, J. L., and Plant, A. G.: Weloganite, a new strontium zirconium carbonate from Montreal Island, Canada, Can. Mineral., 9, 468–477, 1968.
Sheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr. C, 71, 3–8, https://doi.org/10.1107/s2053229614024218, 2015.
Thi, T. T. L., Pobedimskaja, E. A., Nadezhina, T. N., and Khomyakov, A. P.: The crystal structures of alkaline carbonates: barentsite, bonshtedtite and donnayite, Acta Crystallogr. A, 40, C257, https://doi.org/10.1107/S0108767384092291, 1984.
Thi, T. T. L., Pobedimskaja, E. A., Nadezhina, T. N., and Khomyakov, A. P.: Polymorphism of donnayite, (Na,TR)Sr(CO3)2 ⋅ H2O, Vestnik Moskovskogo Universiteta, Geologiya, 47, 60–70, 1992.
Voloshin, A. V., Subbotin, V. V., Yakovenchuk, V. N., Pakhomovskiy, Y. A., Men'shikov, Y. P., Nadezhdina, T. N., and Pushcharovsky, D. Y.: New data on ewaldite, Zapiski Vserossijskogo Mineralogicheskogo Obshchestva, 121, 56–67, 1992.
- Article
(3855 KB) - Full-text XML