the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Grimmite, NiCo2S4, a new thiospinel from Příbram, Czech Republic
Pavel Škácha
Jiří Sejkora
Jakub Plášil
Zdeněk Dolníček
Jana Ulmanová
The new mineral grimmite, NiCo2S4, was found in siderite–sphalerite gangue at the dump of shaft no. 9, one of the mines in the abandoned Příbram uranium and base-metal district, central Bohemia, Czech Republic. The new mineral occurs as rare idiomorphic to hypidiomorphic grains up to 200 µm × 70 µm in size or veinlet aggregates. In reflected light, grimmite is creamy grey with a pinkish tint. Pleochroism, polarising colours and internal reflections were not observed. Reflectance values of grimmite in the air (R %) are 42.5 at 470 nm, 45.9 at 546 nm, 47.7 at 589 nm and 50.2 at 650 nm). The empirical formula for grimmite, based on electron-microprobe analyses (n= 13), is Ni1.01(Co1.99Fe0.06Pb0.01Bi0.01)Σ2.07S3.92. The ideal formula is NiCo2S4; requires Ni 19.26, Co 38.67, and S 42.07; and totals 100.00 wt %. According to the single-crystal X-ray diffraction data (Robs=0.0489), grimmite is cubic, Fd–3m, a=9.3933(9), with V=828.81(14) Å3 and Z=8. The calculated density is 4.96 g cm−3. The strongest reflections of the calculated powder X-ray diffraction pattern [d, Å (I)(hkl)] are 3.3210 (75) (220), 2.7116 (7) (222), 2.3483 (81) (400), 1.9174 (27) (422), 1.6605 (100) (440), 1.4852 (11) (620) and 1.3558 (15) (444). Grimmite is named after Johann Grimm (24 June or 24 July 1805 to 26 June 1874), the former director of the Příbram Mining College. The association of sulfides and sulfarsenides was found with grimmite. Essentially non-zoned coarse-grained siderite encloses idiomorphic crystals and/or aggregates of red sphalerite I and zoned skutterudite-group minerals. Skutterudites (skutterudite, niklskutterudite and ferroskutterudite) are usually strongly corroded and replaced by younger phases. Relics of skutterudite are rimmed by nickeline and later on by gersdorffite with rare domains of glaucodot and arsenopyrite, whereas completely leached parts of skutterudite crystals are filled up by quartz containing small isolated grains and aggregates of pyrite, sphalerite II, grimmite, galena, ullmannite, bismuthinite, parkerite and jaipurite, the latter being rarely enclosed in grimmite.
- Article
(6655 KB) - Full-text XML
-
Supplement
(67 KB) - BibTeX
- EndNote
The nomenclature of thiospinels between ideal polydymite, NiNi2S4, and linnaeite, CoCo2S4, has been ambiguous for a long time. Although original siegenite from its type occurrence in the Siegen District, Westphalia, Germany (Dana, 1850), corresponds to CoNi2S4 and usually siegenite analyses lie on the Ni-rich side of the Ni:Co = 1:1 point, along the Co–Ni join (Biagioni and Pasero, 2014), some authors used the name “siegenite” (Petruk et al., 1969; Imai et al., 1973) or “linnaeite” (Lee et al., 2002) for all compositions between polydymite and linnaeite. Others used the unapproved name “nickellinnaeite” for composition close to the NiCo2S4 (Minčeva-Stefanova and Kostov, 1976; Wagner and Cook, 1999). In the approved nomenclature of the spinel supergroup (Bosi et al., 2019), siegenite is unambiguously defined as a member with the formula CoNi2S4. Complementarily, the phase with the formula NiCo2S4 (present grimmite) is mentioned as a possible new mineral by Hazen et al. (2017).
Synthetically prepared thiospinel NiCo2S4 was studied by Bouchard et al. (1965), who described its unit-cell parameter (a= 9.384(2) Å) and electrical properties, and by Knop et al. (1968), who described its crystal structure (a= 9.3872(7) Å) and magnetic properties. More recently, synthetic NiCo2S4 was intensively investigated for various applications as anodes for lithium-ion batteries, as electroactive materials for micro-SCs and as a bifunctional electrocatalyst for oxygen reduction or evolution reactions (Gao and Huang, 2017; Xia et al., 2015; Shen et al., 2015, and many others).
The new mineral and its name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA 2020-060). Grimmite is named after Johann Grimm (24 June or 24 July 1805–26 June 1874) born in Jáchymov, in the present Czech Republic. He studied at the Štiavnice Mining and Forest Academy in 1825–1830. Later he worked in a mining company in Příbram and he moved to Hungary in 1830, where he later became the main land mining measurer. In 1840 Grimm came back to Příbram to the position of the leading mining and mill administrator. He modernised the mining operations, installed the first steam engines and arranged the long-term stability of the company. In 1850, Grimm became, after Franz Xaver Zippe, the second director of the Příbram Mining College, where he worked until the end of his life. He wrote many books about mining technologies, geology, mining education, and Czech and Hungarian ore districts (Makariová, 2017). The cotype material (two polished sections) is deposited in the mineralogical collection of the Department of Mineralogy and Petrology of the National Museum, Prague, Czech Republic (catalogue number P1P 49/2020), and in the mineralogical collection of the Mining Museum Příbram, Příbram, Czech Republic (catalogue number 3/2020).
One hand-sized specimen of grimmite has been found at the mine dump of the shaft no. 9 – Jerusalem deposit near Příbram. In the period 1951–1991, this mine was one of the shafts open in the shallow parts (to the depth of about 600 m) of the deposit Jerusalem, which belongs to the uranium and base-metal Příbram ore district, central Bohemia, Czech Republic (Komínek, 1995). The GPS coordinates of grimmite occurrence are N, E.
The Příbram ore area (central Bohemia, Czech Republic) is famous for its deposits of base metals as well as for uranium ores. It could be divided into two main ore districts: the base-metal Březové Hory ore district and the complex uranium and base-metal Příbram district. The latter represents the most considerable accumulation of vein-type hydrothermal U ores in the Czech Republic (with the production of 48.432 t of pure U metal) and is comparable to world-class deposits of this type. The hydrothermal U mineralisation of Late Variscan age is related to a 1–2 km wide and almost 25 km long zone formed by a strongly tectonised series of Upper Proterozoic rocks along with the contact with granitoids of the Early Carboniferous Central Bohemian Plutonic Complex (Litochleb et al., 2003). The Příbram uranium and base-metal district can be sub-divided into several ore deposits (also called ore nodes or clusters), among which the most important were Bytíz, Háje and Brod (Ettler et al., 2010). In addition to economic importance, this ore district is also marked by incredible mineralogical diversity (about 200 species known so far), reflecting the occurrence of various types of mineralisation, from the earliest gold-bearing veins through the main uranium and base-metal veins with rich selenide mineralisation locally and also Ag, Ag–Sb, Sb or As bonanzas to post-ore zeolite mineralisations. Grimmite was found there in an arsenide assemblage during a research programme focused on mineralogy and genesis of this ore district, especially selenide (Sejkora et al., 2017; Škácha et al., 2017a, b, 2018), and base-metal and Ag-bearing bonanza-type mineralisations (Sejkora et al., 2019, 2021; Škácha et al., 2019, 2020).
The Jerusalem deposit is located in the central part of the Příbram uranium and base-metal district. The main rocks in the Jerusalem deposit are represented by shales, conglomerates, sandstones, siltstones and mudstones of the upper Proterozoic age in contact with Devonian–Carboniferous granodiorites (Janoušek et al., 2010). The only Jerusalem vein system J1-J38 was mined in the Jerusalem deposit from the surface till the depth of about 1400 m. The NW–SE- or N–S-trending veins usually have steep to sheer dips. The most important uranium content was concentrated in 14 veins, which contain more than 100 t of uranium. The base-metal ores were registered on about only 15 veins and silver-rich ores on the vein B117 (Komínek, 1995).
The new mineral was found in siderite gangue cementing brecciated greenish-black shale. Macroscopically, the ore mineralisation is represented by abundant red sphalerite and aggregates of skutterudite-subgroup minerals up to 1 cm in size. These aggregates show very visible hydrothermal decomposition, which led to the formation of black porous centres.
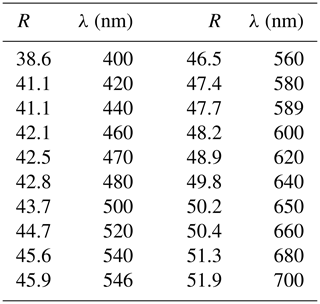
Grimmite occurs as idiomorphic to hypidiomorphic grains up to 200 µm × 70 µm (Fig. 1) or veinlet aggregates 500 µm × 30 µm in size. The mineral is grey with a pinkish tint in colour with a metallic lustre. It is opaque in transmitted light. No cleavage was observed and the fracture is uneven. The calculated density (Z= 8) for the empirical formula is 4.96 g cm−3; for the ideal formula, the density is 4.89 g cm−3. Mohs hardness is assumed at 4 –5 by analogy with other members of the linnaeite subgroup. In reflected light, grimmite is creamy grey with a pinkish tint. Pleochroism, anisotropy under crossed polars and internal reflections were not observed. Reflectance spectra were measured in air with a TIDAS MSP400 spectrophotometer attached to a Leica microscope (50× objective) using a WTiC (Zeiss no. 370) standard, with a square sample measurement field of ca. 8 µm. The results from the 400–700 nm range are given in Table 1 and compared (Fig. 2) with published data for selected members of the linnaeite subgroup (Criddle and Stanley, 1993; Picot and Johan, 1982).
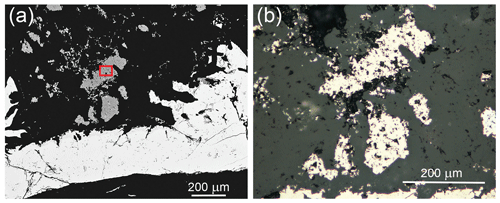
Figure 1(a) Hypidiomorphic grains of grimmite (grey) closely associated with minerals of the skutterudite group (skutterudite and ferroskutterudite) with a thin nickeline rim (all white). BSE photo: the area of extracted fragment for single-crystal study is outlined in red; (b) reflected light photo, partly crossed polarisers.
Chemical analyses were performed using a Cameca SX 100 electron microprobe (National Museum, Prague) operating in wavelength-dispersive mode (25 kV, 20 nA and 1 µm wide beam). The following standards and X-ray lines were used to minimise line overlaps: Ag (Ag Lα), Au (Au Mα), Bi2Se3 (Bi Mβ), CdTe (Cd Lα), Co (Co Kα), CuFeS2 (Cu Kα, S Kα), FeS2 (Fe Kα), HgTe (Hg Mα), Mn (Mn Kα), Ni (Ni Kα), NiAs (As Lα), PbS (Pb Mα), PbSe (Se Lα), PbTe (Te Lα), Sb2S3 (Sb Lα), TlBrI (Tl Lα) and ZnS (Zn Kα). Peak counting time is 20 s for each element and 10 s for each background (detection limit was in the range 0.05–0.20 wt %). Raw intensities were converted to the concentrations of elements using the automatic “PAP” (Pouchou and Pichoir, 1985) matrix-correction procedure.
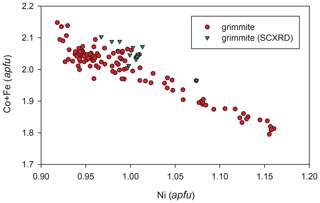
Analytical data for grimmite crystal used for single-crystal study (n = 13) are given in Table 2; these data lead to the empirical formula Ni1.01(Co1.99Fe0.06 Pb0.01Bi0.01)Σ2.07S3.92 on the basis of 7 apfu. The ideal formula is NiCo2S4, which requires Ni 19.26, Co 38.67 and S 42.07 and a total 100.00 wt %. The representative analyses for all grimmite grains (125 point analyses) in cotype samples are given in Table 3. It clearly reveals CoFe−1 substitution with Fe contents up to 0.88 apfu (Fig. 3); no Ni–Fe correlation was observed. The extent of Ni vs. Co+Fe substitution (Fig. 4) is significantly smaller than in the case of Co–Fe. Minor contents of other elements do not exceed 0.10 apfu (As 0.10, Bi 0.04, Cu, Pb, Mn 0.02 and Sb, Se 0.01 apfu).
Table 2Chemical data (wt %) for grimmite grain used for single-crystal X-ray study (n=13).
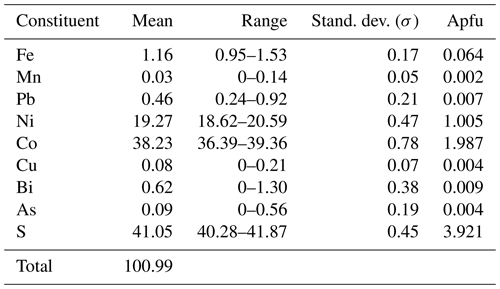
Apfu calculated on the basis of seven atoms.
Table 3Representative analyses (wt %) for grimmite from Příbram.
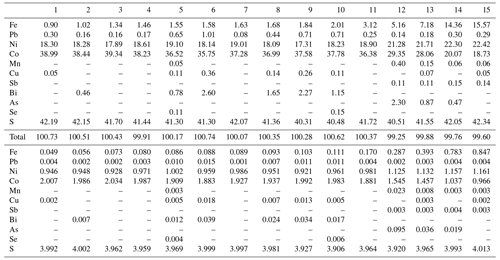
Coefficients of empirical formula were calculated on the basis 7 apfu.
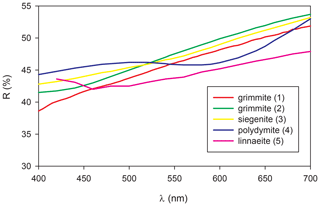
Figure 2Reflectivity curve for grimmite from Příbram (1) compared with published data for Fe-rich grimmite (2) from Outokumpu (Criddle and Stanley, 1993, p. 323, Ni1.07Co1.77Fe0.21S3.95; described as linnaeite nickelian); siegenite (3) from Heinrichssegen mine, Siegen (Criddle and Stanley, 1993, p. 509, Co1.18Ni1.82Fe0.01S3.98); Fe-rich polydymite (4) from Dry Nickel mine (Criddle and Stanley, 1993, p. 448, Ni2.88Fe0.17S3.94); and linnaeite (5 – Picot and Johan, 1982).
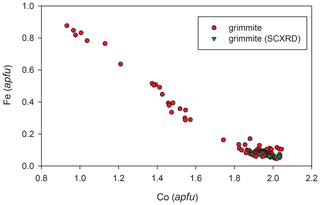
Figure 3The Co vs. Fe (apfu) graph for all studied grimmite grains from Příbram; SCXRD – grain used for single-crystal study.
The powder X-ray diffraction data of grimmite could not be collected due to paucity of material. The calculated (PowderCell 2.3; Kraus and Noltze, 1996) powder diffraction data using the atom coordinates from our crystal structure study are given in Table 4.
Table 4Calculated X-ray powder diffraction data for grimmite.
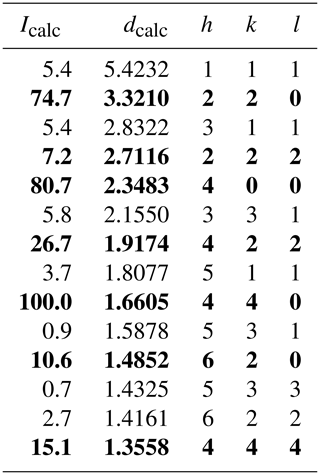
Intensity and dhkl were calculated using the software PowderCell2.3 (Kraus and Nolze, 1996) on the basis of the structural data given in Tables 5 and 6. Only reflections with .7 are listed. The seven strongest reflections are given in bold.
A short prismatic fragment of grimmite, 28 µm × 9 µm × 7 µm in size, extracted from the polished section (Fig. 1, Table 2) analysed using an electron microprobe, was mounted on glass fibre and examined with a Rigaku SuperNova single-crystal diffractometer equipped with an Atlas S2 charge-coupled device (CCD) detector and a microfocus Mo Kα source. The ω rotational scans (frame width of 1.0∘, counting time 1500 s) were adopted for the acquisition of the three-dimensional intensity data. From the total of 1081 reflections, 1078 were taken as independent (without averaging) and 735 considered unique observed with I>3σ(I). Data reduction was performed using CrysAlisPro Version 1.171.39.46 (Rigaku, 2019). The data were corrected for Lorentz factor, polarisation effect and absorption (multi-scan, ABSPACK scaling algorithm; Rigaku, 2019).
According to the single-crystal X-ray data, grimmite is cubic, Fd-3m, a= 9.3933(9), with V= 828.81(14) Å3 and Z= 8. The crystal structure of grimmite has been refined by the software Jana2006 (Petříček et al., 2014) using the structure model for siegenite (Huang and Knop, 1971). The crystal data and the experimental details are given in Table 5, and atom coordinates, atomic displacement parameters and site occupancies are given in Table 6. The interatomic distance (Å) in grimmite for M-S1 (6×) is 2.2635(10) and for T-S1 (4×) is 2.1866(10). Anisotropic displacement parameters are reported in the crystallographic information file that has been deposited in the Supplement.
The unit cell of grimmite is similar to that reported for siegenite by Huang and Knop (1971). The structure of grimmite differs from that of siegenite in the manner that Co occupies Wyckoff site 16c instead of Ni. This is in accordance with the EPMA data. The 8b site in grimmite is occupied by Ni then. We are convinced that Co does not enter the 8b site since the refined value obtained for the refinement with Ni placed solely at 8b returned a value near unity. The refinement involving the restricted (unvaried) occupancy of Co at the tetrahedral site, reflected by the formula Co(NiCo)Σ2S4, also led to similar R values (Robs∼5.5 %), but to a significantly worse goodness of fit (∼2.46 %), in comparison to the final mode we accepted, GOF = 1.46.
Grimmite is the Co-dominant (16c site) and Ni-dominant (8b site) member of the spinel supergroup, thiospinel group, linnaeite subgroup, Strunz class 2/D.01 and Dana class 2.10.1. A comparison of selected data for members of this subgroup is given in Table 7. Grimmite NiCo2S4 is closely chemically related to linnaeite CoCo2S4, siegenite CoNi2S4 and polydymite NiNi2S4 (which is erroneously stated on page 186 of paper spinel supergroup classification – Bossi et al., 2019 – as NiCo2S4). The classification of spinel supergroup minerals is based on the chemical data only (Bossi et al., 2019); therefore Ni–Co thiospinels (series Co3−xNixS4) would be divided into the following four chemical ranges: 0.00 0.50, linnaeite, ideally CoCo2S4; 0.50 1.50, grimmite, ideally NiCo2S4; 1.50 2.50, siegenite, ideally CoNi2S4; and 2.50 3.00, polydymite, ideally NiNi2S4.
Table 7Members of the thiospinel group and linnaeite subgroup. Minerals are listed according to the increasing atomic number of the main cation.
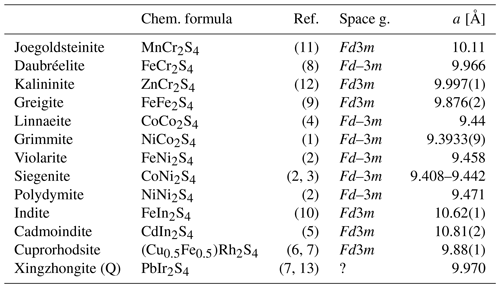
(1) This paper. (2) Riley (1980). (3) Huang and Knop (1971). (4) Buerger and Robinson (1955). (5) Chaplygin et al. (2004). (6) Rudashevsky et al. (1985). (7) Bossi et al. (2019). (8) Lundqvist (1943). (9) Skinner et al. (1964). (10) Genkin and Murav'eva (1963). (11) Isa et al. (2016). (12) Reznickij et al. (1985). (13) Yu et al. (1974); Q – questionable mineral (Bossi et al., 2019).
Mineral phases with chemical composition corresponding to grimmite (Fig. 5) were previously described in various deposits, e.g., from Langis mine, Canada (Petruk et al., 1969); Nippo ore deposit, Japan (Imai et al., 1973); Muzho and Bakura deposits, Bulgaria (Minčeva-Stefanova, 1975); Outokumpu, Finland (Criddle and Stanley, 1993); Elatsite deposit, Bulgaria (Dragov and Petrunov, 1998); or Rainbow hydrothermal field, Mid-Atlantic Ridge (Lee et al., 2002).
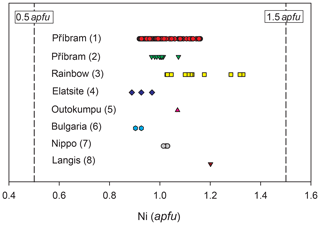
Figure 5Determined range of Ni contents in grimmite. (1) Příbram (this paper). (2) Příbram – grain used for SCXRD study (this paper). (3) Rainbow hydrothermal field, Mid-Atlantic Ridge (Lee et al., 2002). (4) Elatsite deposit, Bulgaria (Dragov and Petrunov, 1998). (5) Outokumpu mining district, Finland (Criddle and Stanley, 1993). (6) Muzho and Bakura deposits, Bulgaria (Minčeva-Stefanova, 1975). (7) Nippo ore deposit, Japan (Imai et al., 1973). (8) Langis mine, Canada (Petruk et al., 1969).
In addition to macroscopically observed skutterudite and sphalerite, a detailed mineralogical study of grimmite-bearing mineralisation also permitted the identification of lower amounts of nickeline, gersdorffite and probably glaucodot, whereas pyrite, arsenopyrite, galena, jaipurite, bismuthinite, ullmannite and parkerite are accessories. The gangue is formed in particular by siderite, whereas calcite and quartz are subordinate, and a silicate with composition close to chlorite is accessory. Essentially non-zoned coarse-grained siderite encloses idiomorphic crystals and/or aggregates of red sphalerite I and zoned skutterudite-group minerals. Skutterudites are usually strongly corroded and replaced by younger phases (Fig. 6a). Relics of skutterudite are rimmed by nickeline (Fig. 6b) and later on by gersdorffite with rare domains of glaucodot and arsenopyrite (Fig. 6c), whereas completely leached parts of skutterudite crystals are filled up by quartz containing small isolated grains and aggregates of pyrite, sphalerite II, grimmite, galena, ullmannite, bismuthinite, parkerite and jaipurite, the latter being rarely enclosed in grimmite (Fig. 6d). The youngest hypogene phase is veinlets and small nests (especially replacing siderite or quartz) of chemically homogeneous calcite. In one case skutterudite enclosed a short monomineral veinlet composed of a chemically heterogeneous porous Ni–Fe–Mg–Al silicate with a composition close to chlorite. The supergene alterations of the studied sample (weathering in dump material) are manifested by further corrosion of skutterudite, partial leaching of carbonates and crystallisation of secondary Zn–Mn–Ni–Co–Fe arsenates.
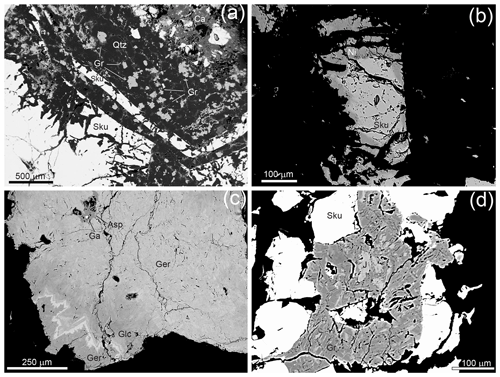
Figure 6Mineral assemblage and textural features of the studied grimmite-bearing sample on BSE images. (a) Zoned skutterudite (Sku) with certain zones selectively replaced by quartz (Qtz) with disseminated grimmite (Gr). Ca – calcite. (b) Corroded skutterudite (Sku) rimmed by nickeline (Ni). (c) Zoned gersdorffite (Ger) with darker domains formed by arsenopyrite (Asp) and glaucodote (Glc) and a bright As-enriched S-poor growth zone. Ga – galena. (d) Skutterudite (Sku) cut by a grimmite (Gr) veinlet containing abundant xenomorphic inclusions of jaipurite (J).
Sphalerite I has low Fe (up to 0.002 apfu) and significant Cd (up to 0.04 apfu), whereas sphalerite II incorporates Ni and Co up to 0.01 apfu and Cd only up to 0.005 apfu. Arsenopyrite contains minor Co (0.004 apfu) and Sb (0.007 apfu); bismuthinite 0.10 apfu Ni and 0.02 apfu Sb; and pyrite minor contents of Ni, Sb and As up to 0.003–0.004 apfu.
Chemical composition of skutterudite-group mineral ranges from skutterudite through nickelskutterudite to ferroskutterudite. Ferroskutterudite, very rare in the world, is the most abundant arsenide in the studied mineral association. At the cation site, it also contains, in addition to prevailing Fe (0.37–0.50 apfu), significant contents of Ni and Co, up to 0.48 and 0.36 apfu, respectively (Table 8). High Fe contents (Fig. 7) were also found in nickelskutterudite (up to 0.47 apfu, Ni 0.48–0.69 and Co up to 0.20 apfu) and in skutterudite (up to 0.44 apfu, Co 0.46–0.59 and Ni up to 0.27 apfu). Similar large Ni–Co–Fe substitution in skutterudite subgroup minerals is described, e.g. from Norilsk ore field (Spiridonov and Gritsenko, 2007) or Dobšiná, Slovakia (Kiefer et al., 2017), and corresponds to the solid-solution field of skutterudite experimentally determined by Roseboom (1962). At the anion site, all members of this subgroup contain S in the range 0.13–0.35 apfu; such increased contents are unusual (Anthony et al., 1990; Spiridonov and Gritsenko, 2007; Spiridonov et al., 2007; Ahmed et al., 2009); similar contents (up to 0.33 apfu S) are described only for samples from Jáchymov (Ondruš et al., 2003).
Table 8Representative analyses (wt %) for members of the skutterudite subgroup from Příbram.
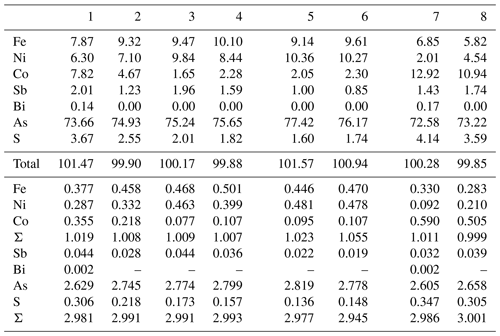
Ferroskutterudite 1–4; nickelskutterudite 5–6; skutterudite 7–8. Coefficients of empirical formula were calculated on the basis of 4 apfu.
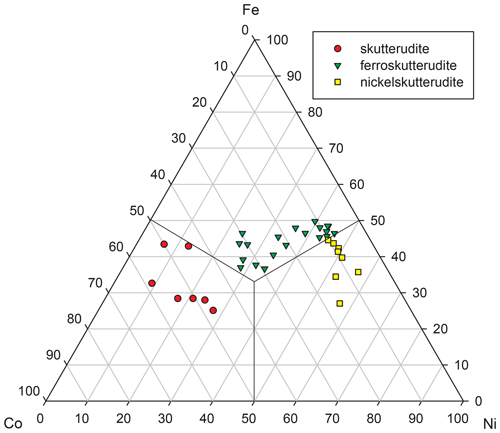
Figure 7Chemical composition (at. units) for members of the skutterudite subgroup from the grimmite-bearing association from Příbram.
The mineral chemistry of weakly anisotropic (to isotropic) sulfarsenides (Ni,Fe,Co)AsS changes rapidly at the micrometre scale. There appear two groups of analyses (Table 9). The composition of the first (Ni-dominant) group varies from gersdorffite with minor Fe and Co contents toward the centre of the Ni–Co–Fe triangle (Fig. 8), and Ni contents correlate positively with As (Fig. 9), as also found by Kiefer et al. (2017) for samples from Dobšiná. The second group is Fe- and Co-dominant members with a Fe : Co ratio around 1:1 (Fig. 8); their compositions are close to that of glaucodot with the ideal formula Co0.5Fe0.5AsS.
Table 9Representative analyses (wt %) for Ni–Fe–Co sulfarsenides from Příbram.
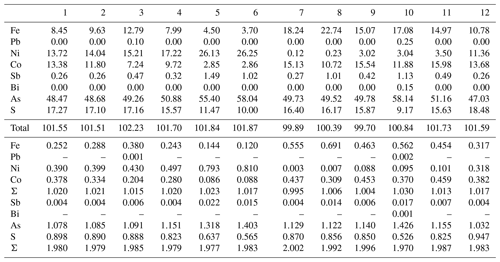
Gersdorffite 1–6; Fe- and Co-dominant members (glaucodot?) 7–12. Coefficients of the empirical formula were calculated on the basis of 3 apfu.
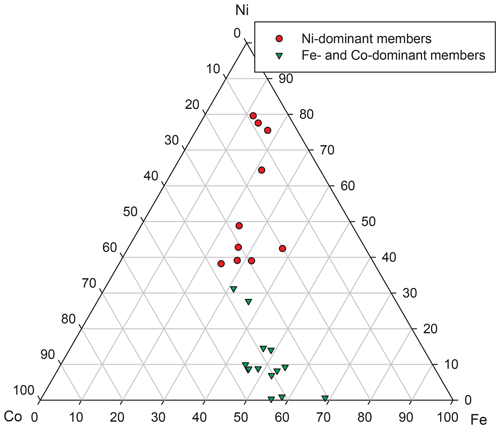
Figure 8Ternary plot Fe–Ni–Co (at. unit) for sulfarsenides from grimmite-bearing association from Příbram.
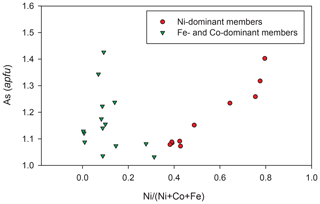
Figure 9The Ni(Ni+Co+Fe) vs. As (apfu) graph for sulfarsenides from the grimmite-bearing association from Příbram.
Nickeline is almost stoichiometric (Table 10) but with very unusual increased Fe contents (0.11–0.16 apfu) and minor Co up to 0.08 apfu. At the anion site, As is partly substituted by S (0.04–0.08 apfu) and Sb (up to 0.02 apfu). Its empirical formula (mean of 21 analyses) on the basis of 2 apfu is (Ni0.81Fe0.14Co0.05)Σ1.00(As0.93S0.06Sb0.01)Σ1.00. Very rare jaipurite (with only a few known occurrences in the world) is close to stoichiometric (Co,Ni,Fe)S (Table 10). In addition to dominant Co, minor contents of Ni and Fe up to 0.18 and 0.08 apfu, respectively, were found. Similar Ni and Fe contents in jaipurite were described by Gertsen et al. (1988). The empirical formula of jaipurite (mean of 13 analyses) on the basis of 2 apfu is (Co0.83Ni0.14Fe0.06)Σ1.03S0.97. The ideal chemical formula for parkerite is reported as Ni3(Bi,Pb)2S2 (Anthony et al., 1990), due to the existence of limited substitution BiPb−1 in the parkerite–shandite series (Fleet, 1973). Parkerite from studied mineral association corresponds to this stoichiometry (Table 10), but it does not contain any Pb; similar Pb-free parkerite was described by Petruk et al. (1969), Groves and Hall (1978), and Sejkora et al. (2009, 2020). Its empirical formula (mean of two analyses) on the basis of 7 apfu is (Ni2.90Co0.03Fe0.02)Σ2.95(Bi1.99Sb0.01)Σ2.00S2.05. Extraordinary Bi-rich ullmannite (Table 10) has an empirical formula of (Ni0.95Co0.01)Σ0.96(Sb0.68Bi0.33)Σ1.01S1.02 on the basis of 3 apfu.
Table 10Representative analyses (wt %) for nickeline, jaipurite, parkerite and ullmannite from Příbram.
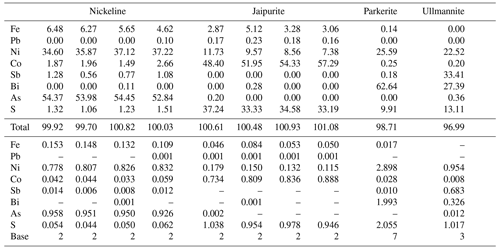
Coefficients of empirical formula were calculated on the given base.
Siderite shows rather uniform chemical composition (Sid63−74 Rdc13−20 Mag10−20 Cal1−3). Calcite is Mn-rich (Cal85−91 Rdc6−11 Sid1−3 Mag1−3), in part with slightly elevated content of Ni (up to 0.7 mol. % NiCO3). Chemical composition of Ni–Fe–Mg–Al silicate with elevated contents of Sb and Zn (up to 0.4 wt % and 1.6 wt % of Sb2O3 and ZnO, respectively) roughly corresponds to Ni-rich Fe-chlorite (chamosite), but its chemical composition was probably partly disturbed (Si excess, low analytical totals) by acid solutions due to weathering.
The economically most important uranium and base-metal mineralisation of the Příbram uranium ore district originated during four main mineralisation stages: (I) siderite–sulfidic; (II) calcite; (III) calcite–uraninite and (IV) calcite–sulfidic (Komínek, 1995). The oldest siderite–sulfidic stage (I) is developed in particular in the southern part of this ore district but on a smaller scale in comparison with the neighbouring Březové Hory district. The younger calcite stages, characterised by notably lower temperatures, are more abundant; calcite generations were used in distinguishing individual mineralisation stages. For the calcite stage (II) pre-ore calcite DK and calcite K1 are characteristic. In the calcite – uraninite stage (III), carrying the main part of the economic uranium mineralisation (uraninite, coffinite and U–bearing anthraxolite), are present calcite types K2–K4. The age of the uranium mineralisation obtained by U–Pb radiometric age determination of two uraninite samples is middle Permian, 275 ± 4 and 278 ± 4 Ma (Anderson, 1987). In the last calcite – sulfidic stage (IV), post-ore calcite K5 appears and Ag, Ag–Sb, Sb and As–Sb bonanza-type accumulations occur.
The paragenetic position, texture and/or chemical composition clearly suggest that siderite and skutterudite present in mineralisation under this study undoubtedly belong to the oldest siderite–sulfide mineralisation stage. Further evolution, however, deviates from the published overall paragenetic scheme (see Žák and Dobeš, 1991; Komínek, 1995). Skutterudite was initially partly dissolved and/or replaced by nickeline, then by gersdorffite and, finally, by quartz-hosted sulfide paragenesis including grimmite and Bi-rich minerals. This evidence implies a late paragenetic position of grimmite and its relation to younger hydrothermal processes possibly related to the same (i.e., siderite–sulfide) or some of the younger mineralisation stages. Unfortunately, a more detailed specification of paragenetic position of the grimmite-bearing mineralising event is not possible because of the specific mineral association of the studied sample and difficulties correlating calcite from the dump sample with the above-mentioned calcite generations, which were defined by means of field observations, textural features and bulk (wet-chemical) composition (Cílek et al., 1984; Komínek, 1995).
The presence of grimmite in association with base-metal sulfides replacing older triarsenides indicates remobilisation processes caused by hydrothermal fluids rich in reduced sulfur. Partial dissolution of earlier Ni–Co arsenides associated with remobilisation of Ni and Co was the likely source of Ni and Co for the formation of grimmite and other Co–Ni enriched sulfides. Phenomena of ore dissolution, remobilisation and replacement were widely detected in the ore veins of the Příbram uranium and base-metal ore district (Sejkora et al., 2019; Škácha et al., 2019; Sejkora et al., 2021). The elevated content of Bi in the studied mineralisation, indicated by the presence of the trace amount of bismuthinite, parkerite and Bi-enriched ullmannite, can also be explained by remobilisation of Bi from earlier gold-bearing quartz veins containing an accessory amount of Bi tellurides (Litochleb et al., 2005, 2006; Sejkora et al., 2019).
The studied mineralisation is characterised by a high activity of iron during the formation of arsenide mineralisation. This took place during crystallisation of triarsenides, giving rise to dominating ferroskutterudite, an otherwise extremely rare mineral worldwide. Similarly, superimposed nickeline incorporated iron in the highest reported concentrations worldwide, to our knowledge. However, explanation of reasons for such unusual iron enrichment cannot be based on pure mineralogical study and need further investigation.
Grimmite, NiCo2S4, a new thiospinel mineral, and its association were described from the Příbram uranium and base-metal district. Grimmite is the Co-dominant (16c site) and Ni-dominant (8b site) member of the spinel supergroup. The host association rich in Fe, Co and Ni includes the skutterudite group, sulfarsenides and arsenides. The presence of grimmite in association with base-metal sulfides replacing older triarsenides indicates remobilisation processes caused by hydrothermal fluids rich in reduced sulfur. Partial dissolution of earlier Ni–Co arsenides associated with remobilisation of Ni and Co was the likely source of Ni and Co for the formation of grimmite and other Co–Ni-enriched sulfides.
Crystallographic data for grimmite are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-33-175-2021-supplement.
PŠ found the mineral, evaluated part of the EPMA analyses and provided valuable information. JS, JP, ZD and JU analysed the material (structure determinations were done by JP, EPMA by ZD and JU; optical properties by JS). JS and ZD interpreted the results and wrote the paper, with help from PŠ and JP.
The authors declare that they have no conflict of interest.
The helpful comments of the anonymous members of the Commission on New Minerals, Nomenclature and Classification I.M.A are greatly appreciated.
The research was financially supported by the project 19-16218S of the Czech Science Foundation for Pavel Škácha, Jiří Sejkora, Zdeněk Dolníček and Jana Ulmanová. We acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110) for the financial support of the sample measurements.
This paper was edited by Sergey Krivovichev and reviewed by Yves Moëlo and Ulf Hålenius.
Ahmed, A. H., Arai, S., and Ikenne, M.: Mineralogy and paragenesis of the Co-Ni arsenide ores of Bou Azzer, Anti-Atlas, Morocco, Econ. Geol., 104, 249–266, 2009.
Anderson, E. B.: Isotopic-geochronological investigation of the uranium mineralization of Czechoslovaki, Unpublished Czechoslovak Uranium Industry Report 1962-87, 1987.
Anthony, J. W., Bideaux, R. A., Bladh, K. W., and Nichols, M. C.: Handbook of Mineralogy: Volume I. Elements, Sulfides, Sulfosalts, Mineral Data Publishing, Tucson, Arizona, 1–588, 1990.
Biagioni, C. and Pasero, M.: The systematics of the spinel-type minerals: An overview, Am. Mineral., 99, 1254–1264, 2014.
Bossi, F., Biagioni, C., and Pasero, M.: Nomenclature and classification of the spinel supergroup, Eur. J. Mineral., 31, 183–192, https://doi.org/10.1127/ejm/2019/0031-2788, 2019.
Bouchard, R. J., Russo, P. A., and Wold, A.: Preparation and electrical properties of some thiospinels, Inorg. Chem., 4, 685–688, 1965.
Buerger, M. J. and Robinson, D. W.: The Crystal Structure and Twinning of Co2S3, P. Natl. Acad. Sci. USA, 41, 199–203, 1955.
Chaplygin, I. V., Mozgova, N. N., Bryzgalov, I. A., and Mokhov, A. V.: Cadmoindite, CdIn2S4, a new mineral from Kudriavy volcano, Iturup isle, Kurily islands, Zap. Vserossij. Mineralog. Obsh., 133, 21–27, 2004.
Cílek, V., Prokeš, S., Škubal, M., Hladíková, J., Šmejkal, V., and Žák, K.: Geochemistry of hydrothermal carbonates of the Příbram uranium deposit, Vlastivěd. Sbor. Podbrdska, 26, 79–102, 1984.
Criddle, A. J. and Stanley, C. J.: Quantitative data file for ore minerals, 3rd Edn., Chapman & Hall, London, 1993.
Dana, J. D.: Siegenite, in: A system of Mineralogy, 3rd Edn., 687 pp., Putnam, New York, 1850.
Dragov, P. and Petrunov, R.: Composition of thiospinel minerals from Elatsite porphyry-copper deposit, Geochem. Mineral. Petrolog., 33, 25–28, 1998.
Ettler,V., Sejkora, J., Drahota, P., Litochleb, J., Pauliš, P., Zeman, J., Novák, M., and Pašava, J.: Příbram and Kutná Hora mining districts – from historical mining to recent environmental impact, in: IMA 2010, Budapest, Acta Mineral.-Petrogr., Field Guide Series, 7, 1–23, 2010.
Fleet, M. E.: The crystal structure of parkerite (Ni3Bi2S2), Am. Mineral., 58, 435–439, 1973.
Gao, Y. P. and Huang, K. J.: NiCo2S4 materials for supercapacitor applications, Chemistry – An Asian Journal, 12, 1969–1984, 2017.
Genkin, A. D. and Murav'eva, I. V.: Indite and dzhalindite, new indium minerals, Zap. Vserossij. Mineralog. Obsh., 92, 445–457, 1963.
Gertsen, L. Y., Kotelnikov, P. Y., and Yeremeyeva, Y. Y.: Second jaipurite find in the world, Trans. (Dokl.) USSR Acad. Sci., Earth Sci. Sect., 303, 157–160, 1988.
Groves, D. I. and Hall, S. R.: Argentian pentlandite with parkerite, joseite A and the probable Bi-analogue of ullmannite from Mount Windarra, Western Australia, Can. Mineral., 16, 1–7, 1978.
Hazen, R. M., Hystad, G., Golden, J. J., Hummer, D. R., Liu, C., Downs, R. T., Morrison, S. M., Ralph, J., and Grew, E. S.: Cobalt mineral ecology, Am. Mineral., 102, 108–116, 2017.
Huang, C. H. and Knop, O.: Chalkogenides of the transition elements. VIII An X-ray and neutron diffraction study of spinel CoNi2S4, Can. J. Chem., 40, 598–602, 1971.
Imai, N., Mariko, T., and Shiga, Y.: Siegenite from the Nippo ore deposit of the Kamaishi mine, Iwate Prefecture, Japan, Mining Geol., 23, 347–354, 1973.
Isa, J., Ma, C., and Rubin, A. E.: Joegoldsteinite: A new sulfide mineral (MnCr2S4) from the Social Circle IVA iron meteorite, Am. Mineral., 101, 1217–1221, 2016.
Janoušek, V., Wiegand, B. A., and Žák, J.: Dating the onset of Variscan crustal exhumation in the core of the Bohemian Massif: new U–Pb single zircon ages from the high-K calc-alkaline granodiorites of the Blatná suite, Central Bohemian Plutonic Complex, J. Geol. Soc., 167, 347–360, 2010.
Kiefer, S., Majzlan, J., Chovan, M., and Števko, M.: Mineral compositions and phase relations of the complex sulfarsenides and arsenides from Dobšiná (Western Carpathians, Slovakia), Ore Geol. Rev., 89, 894–908, 2017.
Knop, O., Reid, K. I. G., Sutarno, and Nakagawa, Y.: Chalkogenides of the transition elements. VI. X-Ray, neutron, and magnetic investigation of the spinels Co3O4, NiCo2O4, Co3S4, and NiCo2S4, Can. J. Chem., 46, 3463–3476, 1968.
Komínek, J.: Geology of the Příbram district and its broader surroundings, in: Final report of the Příbram district, 2nd part, Unpublished MS, DIAMO State Enterprise, 1995.
Kraus, W. and Nolze, G.: POWDER CELL – a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns, J. Appl. Crystallogr., 29, 301–303, 1996.
Lee, S. Y., Watanabe, M., Hoshino, K., Oomori, T., Fujioka, K., and Rona, P. A.: First report of linnaeite (Co3S4) and millerite (NiS) from active submarine hydrothermal deposits: Rainbow hydrothermal field, Mid-Atlantic Ridge at 36∘14' N, N. Jb. Mineral. Mh., 2002, 1–21, 2002.
Litochleb, J., Černý, P., Litochlebová, E., Sejkora, J., and Šreinová, B.: The deposits and occurrences of mineral raw materials in the Střední Brdy Mts. and the Brdy piedmont area (Central Bohemia), Bull. Mineral.-Petrolog. Odd. Nár. Muz. (Praha), 11, 57–86, 2003.
Litochleb, J., Sejkora, J., and Škácha, P.: Tsumoite (BiTe) from the gold-bearing quartz veins from Bytíz – Staré hory near Příbram, Bull. Mineral.-Petrolog. Odd. Nár. Muz. (Praha), 13, 150–153, 2005.
Litochleb, J., Sejkora, J., and Šrein, V.: The Au-Ag-Sb-Bi-Te mineralization from the deposit Bytíz (Mine 19), the Příbram uranium-polymetallic ore district, Czech Republic, Mineral. Polon.-Spec. Pap., 28, 133–135, 2006.
Lundqvist, D.: Crystal structure of daubréelite, Arkiv Kemi, Mineral. Geol., 17B, 1–4, 1943.
Makariová, M. (Ed.): Biographic dictionary of the Bohemian lands, Academia, 744 pp., 2017.
Minčeva-Stefanova, J.: Nickelian cobaltite, cuprosiegnite, nickelian carrollite and cobaltian gersdorffite from the stratiform polymetallic deposits in the western Stara Planina Mountains, Geochem. Mineral. Petrolog., 3, 31–51, 1975.
Minčeva-Stefanova, J. and Kostov, I.: On siegenite and the “miscibility” between linnaeite and polydymite, Geochem. Mineral. Petrolog., 4, 35–56, 1976.
Ondruš, P., Veselovský, F., Gabašová, A., Hloušek, J., Šrein, V., Vavřín, I., Skála, R., Sejkora, J., and Drábek, M.: Primary minerals of the Jáchymov ore district, J. Czech Geol. Soc., 48, 19–147, 2003.
Petříček, V., Dušek, M., and Palatinus, L.: Crystallographic computing system Jana2006: general features, Z. Kristallogr., 229, 345–352, 2014.
Petruk, W., Harris, D. C., and Stewart, J. M.: Langisite, a new mineral, and the rare minerals cobalt pentlandite, siegenite, parkerite and bravoite from the Langis mine, Cobalt-Gowganda area, Ontario, Can. Mineral., 9, 597–616, 1969.
Picot, P. and Johan, Z.: Atlas of ore minerals, B.R.G.M., Elsevier, 1982.
Pouchou, J. L. and Pichoir, F.: “PAP” (πρZ) procedure for improved quantitative microanalysis, in: Microbeam Analysis, edited by: Armstrong, J. T., San Francisco Press, California, 104–106, 1985.
Reznickij, L. Z., Skl'arov, E. V., and Ustschapovskaya, Z. F.: Kalininite ZnCr2S4 – a new natural sulphospinel, Zap. Vserossij. Mineralog. Obsh., 114, 622–627, 1985.
Rigaku: CrysAlis CCD and CrysAlis RED. Rigaku-Oxford Diffraction Ltd, Yarnton, Oxfordshire, UK, 2019.
Riley, F. J.: Ferroan carrollites, cobaltian violarites, and other members of the “linnaeite group” (Co,Ni,Fe,Cu)3S4, Mineral. Mag., 43, 733–739, 1980.
Roseboom, E. H.: Skutterudites (Co, Ni, Fe)As3−x: Composition and cell dimensions, Am. Mineral., 47, 310–327, 1962.
Rudashevsky, N. S., Men'shikov, Y. P., Mochalov, A. G., Trubkin, N. V., Shumskaya, N. I., and Zhdanov, V. V.: Cuprorhodsite CuRh2S4 and cuproiridsite CuIr2S4 – new natural thiospinels of platinum-group elements, Zap. Vserossij. Mineralog. Obsh., 114, 187–195, 1985.
Sejkora, J., Litochleb, J., and Süsser, C.: The occurrence of parkerite at the uranium ore district Horní Slavkov (Czech Republic), Bull. Mineral.-Petrolog. Odd. Nár. Muz. (Praha), 17, 29–32, 2009.
Sejkora, J., Škácha, P., Laufek, F., and Plášil, J.: Brodtkorbite, Cu2HgSe2, from Příbram, Czech Republic: crystal structure and description, Eur. J. Mineral., 29, 663–672, https://doi.org/10.1127/ejm/2017/0029-2647, 2017.
Sejkora, J., Škácha, P., and Dolníček, Z.: Ag-Bi-Hg mineralization from the deposit Brod, uranium and base-metal ore district Příbram (Czech Republic), Bull. Mineral. Petrolog., 27, 259–268, 2019.
Sejkora, J., Litochleb, J., Novák, M., Cícha, J., and Dolníček, Z.: Nickel-(Bi, Ag) sulphide mineralization from NYF Vepice pegmatite, Milevsko pluton, southern Bohemia (Czech Republic) – a reflection of the parental granite chemistry, J. Geosci., 65, 187–199, 2020.
Sejkora, J., Škácha, P., Plášil, J., Dolníček, Z. and Ulmanová, J.: Hrabákite, Ni9PbSbS8, a new member of the hauchecornite group from Příbram, Czech Republic, Mineral. Mag., https://doi.org/10.1180/mgm.2021.1, online first, 2021.
Shen, L., Wang, J., Xu, G., Li, H., Dou, H., and Zhang, X.: NiCo2S4 nanosheets grown on nitrogen-doped carbon foams as an advanced electrode for supercapacitors, Adv. Energy Mater., 5, 1400977, https://doi.org/10.1002/aenm.201400977, 2015.
Škácha, P., Sejkora, J., and Plášil, J.: Příbramite, CuSbSe2, the Se-analogue of chalcostibite, a new mineral from Příbram, Czech Republic, Eur. J. Mineral., 29, 653–661, https://doi.org/10.1127/ejm/2017/0029-2623, 2017a.
Škácha, P., Sejkora, J., and Plášil, J.: Selenide mineralization in the Příbram uranium and base-metal district (Czech Republic), Minerals, 7, 91, https://doi.org/10.3390/min7060091, 2017b.
Škácha, P., Sejkora, J., and Plášil, J.: Bytízite, a new Cu-Sb selenide from Příbram, Czech Republic, Mineral. Mag., 82, 199–209, 2018.
Škácha, P., Sejkora, J., and Dolníček, Z.: Cu-Ag-Sb-As mineralization from the Milín deposit, uranium and base-metal ore district Příbram (Czech Republic), Bull. Mineral. Petrolog., 27, 419–426, 2019.
Škácha, P., Sejkora, J., Plášil, J., and Makovicky, E.: Pošepnýite, a new Hg-rich member of the tetrahedrite group from Příbram, Czech Republic, J. Geosci., 65, 173–186, 2020.
Skinner, B. J., Erd, R. C., and Grimaldi, F. S.: Greigite, the thio-spinel of iron; a new mineral, Am. Mineral., 49, 543–555, 1964.
Spiridonov, E. M. and Gritsenko, Y. D.: Ferroskutterudite, nickelskutterudite, and skutterudite from the Norilsk ore field, New Data on Minerals Moscow, 42, 16–27, 2007.
Spiridonov, E. M., Gritsenko, Y. D., and Kulikova, I. M.: Ferroskutterudite (Fe,Co)As3: a new mineral species from the dolomite-calcite veins of the Noril'sk ore field, Dokl. Earth Sci., 417, 1278–1280, 2007.
Wagner, T. and Cook, N. J.: Carrollite and related minerals of the linnaeite group; solid solutions and nomenclature in the light of new data from the Siegerland District, Germany, Can. Mineral., 37, 545–558, 1999.
Xia, C., Li, P., Gandi, A. N., Schwingenschlögl, U., and Alshareef, H. N.: Is NiCo2S4 really a semiconductor?, Chem. Mater., 27, 6482–6485, 2015.
Yu, T. H., Lin, S. J., Chao, P., Fang, C. S., and Huang, C. S.: A preliminary study of some new minerals of the platinum-group and another associated new one in platinum-bearing intrusions in a region of China, Acta Geol. Sin., 2, 202–218, 1974.
Žák, K. and Dobeš, P.: Stable isotopes and fluid inclusions in hydrothermal deposits: the Příbram ore region, Rozpr. Českoslov. Akad. věd, Ř. matem., přír. Věd, 101, 1–109, 1991.
- Abstract
- Introduction
- Occurrence
- Appearance and physical properties
- Chemical composition
- Crystallography
- Crystal chemistry and relationship with known (Co, Ni) thiospinels
- Associated minerals
- Remarks to the origin of grimmite-bearing mineralisation
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Occurrence
- Appearance and physical properties
- Chemical composition
- Crystallography
- Crystal chemistry and relationship with known (Co, Ni) thiospinels
- Associated minerals
- Remarks to the origin of grimmite-bearing mineralisation
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement