Tancaite-(Ce), ideally FeCe(MoO4)3 ● 3H2O: description and average crystal structure
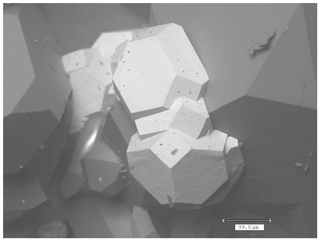
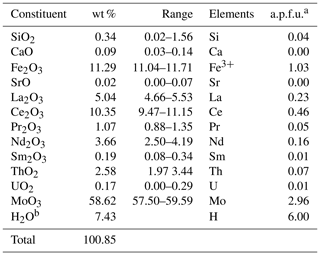
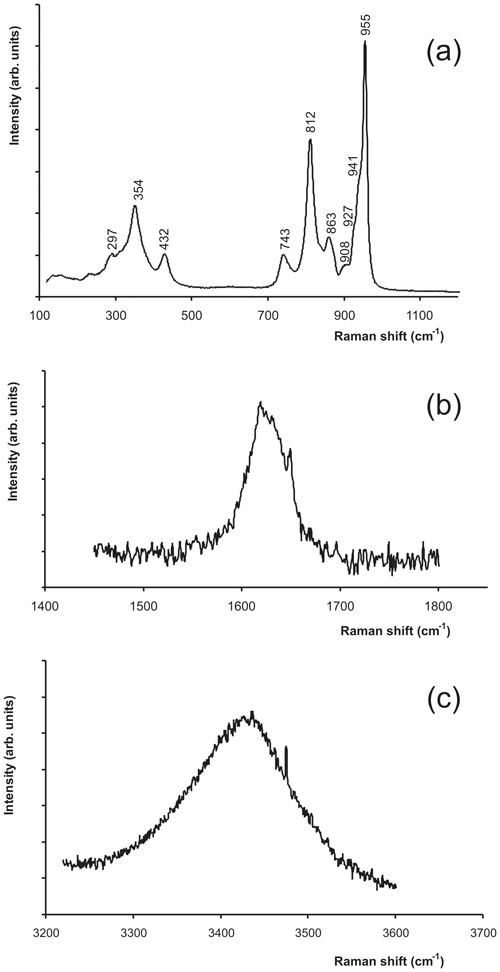
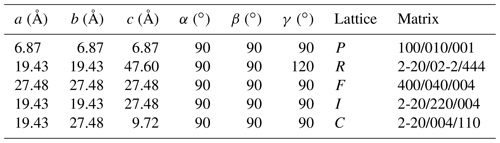
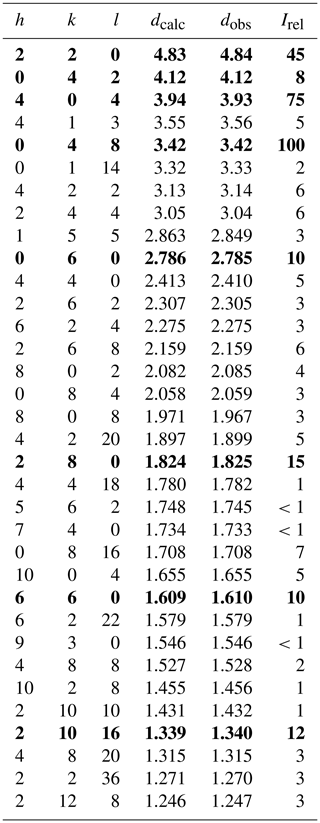
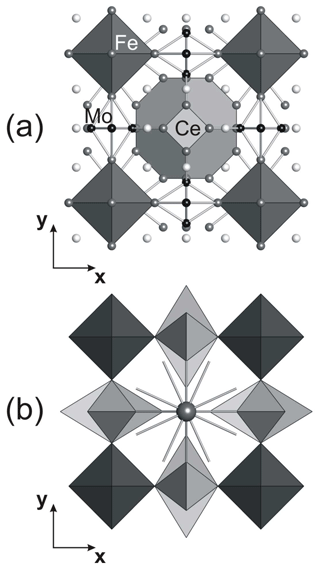
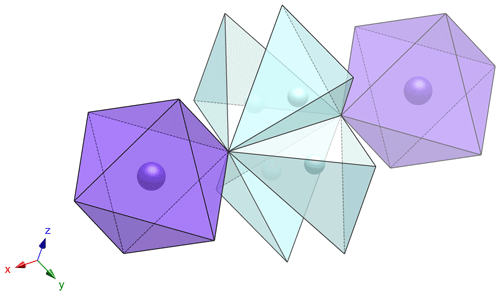
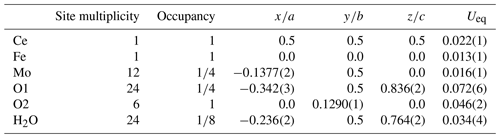
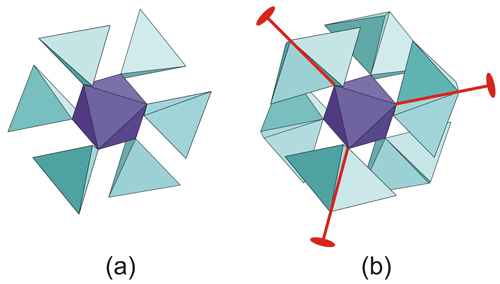
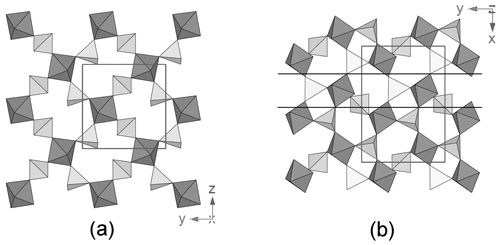
Tancaite-(Ce), ideally FeCe(MoO4)3•3H2O, is a new mineral occurring within cavities in the quartz veins which cut the granite at Su Seinargiu, Sarroch (CA), Sardinia, Italy. It is a secondary mineral formed in the oxidation zone of a sulfide ore vein. Associated minerals are quartz, muscovite, molybdenite, pyrite, and a mendozavilite-like phase. Tancaite-(Ce) is red or pale brown in colour, with a vitreous to adamantine lustre. Electron microprobe analyses give (wt %) SiO2 0.34, CaO 0.09, Fe2O3 11.29, SrO 0.02, La2O3 5.04, Ce2O3 10.35, Pr2O3 1.07, Nd2O3 3.66, Sm2O3 0.19, ThO2 2.58, UO2 0.17, MoO3 58.62, and H2O (calculated) 7.43, with a sum of 100.85, from which the empirical formula is calculated. The empirical formula (Ce0.46La0.23Nd0.16Pr0.05Sm0.01U0.01Th0.07)Σ=0.99(Mo2.96Si0.04)Σ=3.00O12•3H2O can be simplified as Fe3+(REE)(MoO4)3•3H2O and idealized as FeCe(MoO4)3•3H2O. The presence of H2O was confirmed by micro-Raman spectrometry (stretching and bending vibrations of O–H). The calculated density is 3.834 g cm−3. The X-ray diffraction pattern of tancaite-(Ce) is characterized by a set of strong reflections, which point to a cubic subcell with a=6.870(1) Å and space group , plus a set of superstructure reflections. Tancaite-(Ce) displays a new structure type not previously reported in natural and synthetic molybdates. By considering only the strong reflections, it was possible to solve and refine its average structure (R1=0.038 for 192 unique reflections with I>2σ(I)). The crystal structure consists of FeO6 octahedra centred at the origin of the cubic subcell and linked together through MoO4 tetrahedra by corner sharing. The Mo-centred tetrahedra are statistically distributed in four symmetry-related positions, with one-fourth occupancy. In the centre of the cubic unit cell the REE cations exhibit a 6+3 coordination, bonding six oxygen atoms and three H2O molecules, each of them being disorderly distributed in four symmetry-related positions. One of the possible supercells, with a 48-fold volume with respect to the primitive cubic small subcell, corresponded to a rhombohedral lattice, with a≈19.43 and c≈47.60 Å in the hexagonal setting. Several unsuccessful trials were performed to solve the real crystal structure of tancaite, by indexing the additional superstructure reflections and using their intensities to refine an ordered structural model. The new mineral has been approved by the IMA CNMNC (no. 2009-097). The name comes from Giuseppe Tanca, an Italian amateur mineralogist, who discovered the mineral and gave it to us for studying.