the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The oxidation of Fe in riebeckite at 0.7 GPa
Giancarlo Della Ventura
Roberta Oberti
Valeria Misiti
Francesco Radica
Gunther J. Redhammer
Simone Bernardini
Massimo Boiocchi
Boriana Mihailova
At high temperatures, riebeckite, ideally Na2(FeFe)Si8O22(OH)2, undergoes significant crystal-chemical rearrangement in response to the following reaction: Fe2+ + OH− → Fe3+ + O2− + e− + H+, leading to the development of charge carriers (hopping electrons and delocalized H+ ions) and to the release of H2O. The process of oxidation of Fe and dehydrogenation thus has important implications for planetary-scale phenomena, including electrical conductivity, water cycling, seismicity, volcanism, and ore generation, particularly in subduction zones.
In this work, we address the effect of pressure on the oxidation of Fe in riebeckite, a process that is irreversible for temperatures above ∼ 377 °C at 1 atm pressure, under oxidizing conditions. Single crystals of riebeckite were annealed using a piston-cylinder apparatus at temperatures (T) in the 600–750 °C range under a constant pressure (P) of 0.7 GPa and oxygen fugacity () close to Ni-NiO (NNO). The crystal-chemical response of the amphibole to these P–T– conditions was analyzed using a combination of X-ray diffraction and spectroscopic methods, including Mössbauer, Raman, and Fourier transform infrared (FTIR) spectroscopy.
At 0.7 GPa, there is no oxidation of Fe in riebeckite. The stability of the amphibole structure extends at least up to 750 °C, without any transition to oxo-riebeckite and hence without any release of H+.
Since the conditions of our experiments replicate those of rocks at pressures corresponding to ∼ 25 km depth, our findings further support the concept that thermally activated electron hopping significantly contributes to the electrical conductivity of lithospheric rocks, and we suggest that both conductivity and water cycling in the Earth's interior may be active over a broader P–T range (and thus at grater depths) than previously considered.
- Article
(4224 KB) - Full-text XML
-
Supplement
(261 KB) - BibTeX
- EndNote
At high temperature, iron-rich amphiboles undergo crystal-chemical rearrangement in response to the oxidation of Fe, which is schematically represented by the following reaction (Addison et al., 1962a; Phillips et al., 1988; Della Ventura et al., 2018a, Bernardini et al., 2024):
This is probably one of the most studied processes involving H+ because of its implications in petrology, geophysics, and materials science. Some of the issues related to this reaction have been addressed only in recent years due to the integration of different analytical methods (Oberti et al., 2016, 2018; Della Ventura et al., 2018a, b, 2022, 2023, 2024a). These studies have shown that the oxidation of Fe in amphiboles is a dynamic, complex, and multistep phenomenon, whose crystal-chemical features are strongly affected by oxygen fugacity () (Della Ventura et al., 2005, 2023).
Amphiboles are among the main constituents of metamorphic rocks in subduction zones (Wang et al., 2012; Hu et al., 2018). Alkali amphiboles in particular are ubiquitous phases in glaucophane schists that formed at high pressure in regions of tectonic activity (e.g., Ernst, 1963a; Deer et al., 1997; Sisson et al., 1997; Howe et al., 2018; Bang et al., 2021); therefore, these minerals strongly affect the physical properties of the mid-crust to upper mantle, being potential carriers of both electrical conductivity (e.g., Hu et al., 2018) and water at depth (e.g., Schmidt and Poli, 1998; Mandler and Grove, 2016). Although various hydrous minerals (e.g., lawsonite, epidote, phlogopite, and chlorite) are responsible for the transport of fluids at greater depths during high-pressure metamorphism, amphiboles are the main water sink, accounting for as much as 20 wt %–60 wt % of the metamorphosed oceanic crust (Schmidt and Poli, 1998).
Amphiboles (Fig. 1 displays a sketch of the structure showing the site nomenclature) have the following general formula (Hawthorne and Oberti, 2007) AB2C5T8O22W2, where
Riebeckite, ideally A□BNa2C(FeFe)TSi8O(OH)2, transforms into oxo-riebeckite, ideally A□BNa2C(Fe2+Fe)TSi8OO2, when heated in the presence of external O2 (Della Ventura et al., 2023). The oxidation of Fe involves only minor structural adjustments, such as kinking of the ribbons of tetrahedra to accommodate the smaller size of the octahedra occupied by Fe3+. Notably, this transition significantly enlarges the stability field of the amphibole, with oxo-riebeckite remaining stable up to ca. 900 °C (Oberti et al., 2018). In the absence of external oxygen, the oxidation/dehydration process does not occur, and only a cation rearrangement is observed, yielding what Della Ventura et al. (2023) describe as a “CR3+-disordered riebeckite”. For T>750 °C, this amphibole breaks down, showing a stability limit about 150 °C lower than that of oxo-riebeckite.
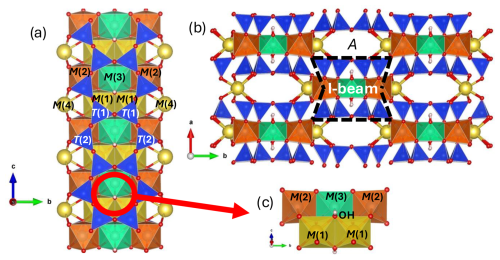
Figure 1The (C2/m) amphibole structure projected (a) along the [001] crystallographic direction with the cationic T and M sites indicated, and (b) perpendicular to the [001] axis, where the I beam and the A site are made evident. In (c), an enlargement of the cationic environment around the O–H group is displayed. Drawings were made with VESTA (Momma and Izumi, 2008).
The most important issue of the oxidation/dehydrogenation process is that it involves the development of charge carriers (both electrons and H+ ions), before the final release of water in the surroundings (Bernardini et al., 2023, 2024; Della Ventura et al., 2024a, 2024b). This process has significant implications for Earth-scale phenomena, including anomalous high conductivity, seismicity, volcanism, and ore generation, particularly in convergent geodynamic domains. Note that in the presence of external molecular O2, the release of water occurs at much lower temperatures than under reducing conditions (Della Ventura et al., 2023).
Oberti et al. (2018) have shown that, for T>600 °C and at ambient pressure in air, riebeckite undergoes irreversible oxidation of Fe coupled with H+ loss. In this work, we examine the role of pressure on the oxidation of Fe in riebeckite by applying a combination of analytical methods, including structure refinements to single-crystal X-ray diffraction (XRD) data and FTIR, Raman, and Mössbauer spectroscopies. Our investigation focuses on samples from the same batch used by Della Ventura et al. (2018a) and Oberti et al. (2018) for their studies at 1 atm. Here, we characterized ex situ the crystals after annealing at four different temperatures (600, 650, 700, and 750 °C) under a constant pressure of 0.7 GPa and close to NNO.
Single crystals of riebeckite were treated at 0.7 GPa using an end-loaded piston-cylinder apparatus at INGV (Rome, Italy) under oxidizing conditions close to the oxygen fugacity of the Ni-NiO (NNO) buffer. The crystal-chemistry of the used sample is fully described in Susta et al. (2018) and Oberti et al. (2018); briefly, its chemical composition is A(□0.9K0.06Na0.04)B(Na1.82Ca0.13FeC[M(1)(FeMg0.16)M(2)(FeMg0.04FeAl0.10Ti0.01) M(3)(FeMg0.06Mn0.05)]T(Si7.97Al0.03)O(OH)1.90F0.10, which is very close to that of end-member riebeckite.
Several amphibole grains (average size 300–400 µm in length and 100–200 µm across) were sealed into 15 mm length Pt tubes surrounded by powdered MgO to prevent the tube from collapsing under pressure; 10 wt % of deionized water was added, and the samples were run at 600 °C (sample RHP3), 650 °C (RHP5), 700 °C (RHP6), and 750 °C (RHP7) for a duration of 2 h under a constant pressure of 0.7 GPa. Capsules were positioned into a 19.1 mm NaCl–crushable alumina–pyrophyllite–pyrex assembly, which produces an close to NNO+2 (Kushiro, 1990; Kawamoto and Hirose, 1994).
Annealed single crystals (dark green in color and irregular prismatic in morphology) from runs RHP5, RHP6, and RHP7 were studied via single-crystal XRD done at room temperature. Unit-cell parameter (ucp) measurements and intensity data collections were done at CNR-IGG in Pavia using a Philips PW1100 diffractometer operating with Mo Kα radiation at 30 mA and 55 kV. For each dataset, two quadrants of intensity data were collected (±h, ±k, +l) by the scan in the 2–30° θ range. Scan widths and scan speeds are 2.8° θ and 0.14° s−1, 2.4° θ and 0.06° s−1, and 2.7° θ and 0.09° s−1 for 1345, 1346, and 1347 samples, respectively. The intensities of three standard reflections were monitored every 400 measured reflections, and no significant variation in beam intensity was registered during the data collection. Raw intensities of reflections were obtained by measuring step-scan profiles and integrating by the Lehmann and Larsen (1974) method, as modified by Blessing et al. (1974). Raw intensities were corrected for Lorentz and polarization effects as well as for absorption using the ψ-scan method of North et al. (1968). Final accurate ucp values were obtained from a least-squares procedure based on 60 dhkl values, each measured from the positions of reflection pairs at ±θ in the range 2–30° θ.
Structure refinement (SREF) was done using a program written in Pavia (Cannillo et al., 1983), which allows for the use of neutral versus ionized scattering curves at the sites where solid solution does not occur, as well as combinations of ionized scattering curves in all other sites (Hawthorne et al., 1995; Oberti et al., 2017). Full matrix least-squares refinement on F was done until convergence without using any weight or chemical constraint. Relevant parameters of data collections and refinement procedures are reported in Table 1. The evolution of the geometric parameters (atom coordinates, bond distances, and angles) relevant to the present discussion are reported in Table 2. Atom coordinates and displacement parameters are reported in Table S1 (in the Supplement). All other refinement details and the observed structure factors have been deposited as CIF files.
Table 1Crystal-structure refinement data for the riebeckite samples annealed at 0.7 GPa. Data for the untreated riebeckite crystal (from Oberti et al., 2018, IGG-Pv code 1272) from the same batch used here are also listed for comparison. Note that ss represents site scattering, and epfu represents electron per formula unit
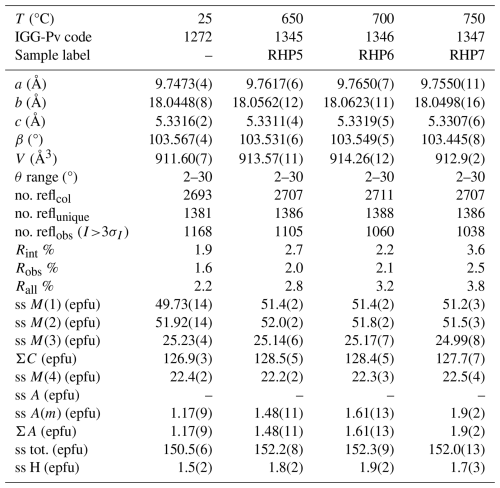
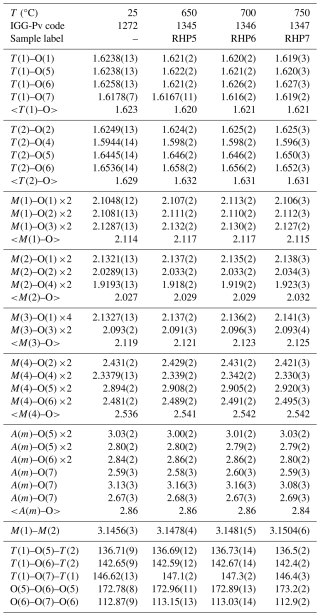
Table 2Selected interatomic distances (Å) and interatomic angles (°) in the double-chain of tetrahedra for the annealed riebeckite samples. Sample codes are the same as in Table 1.
Single-crystal FTIR spectra were collected at Istituto Nazionale di Fisica Nucleare (INFN), Frascati (Rome), using a Bruker Hyperion 3000 FTIR microscope, equipped with a 15× Schwarzschild objective and a mercury cadmium telluride (MCT) liquid nitrogen (LN2)-cooled detector. The microscope was attached to a Vertex 70v optical bench equipped with a KBr beam splitter and a Globar IR source. The beam cross section was restricted to the size of the sample (∼ 100 × 30 × 20 µm3) with a rectangular aperture. The instrumental resolution was set at 2 cm−1, and 128 spectra were co-added for both mineral and background. Additional FTIR polarized spectra were acquired for sample RHP3 using a gold wire grid and a ZnSe substrate FTIR polarizer.
Raman spectra were acquired at the University of Hamburg with a Horiba T64000 triple-monochromator system operating in a subtractive regime and equipped with a Symphony LN2-cooled CCD detector and an Olympus BX41 confocal microscope. Polarized Raman scattering in the ranges 15–1215 and 3450–3800 cm−1 was measured in backscattering geometry, using the 514.532 nm line of a Coherent 90C Fred Ar+ laser. The laser beam was focused to a spot of linear size of ∼ 2 µm on raw natural surfaces of single crystals. The effective penetration depth was on the order of several micrometers. The measured spectra were evaluated and fitted with pseudo-Voigt functions to determine the peak positions, full width at half maximum (FWHM), and integrated intensities, following the procedure described in detail by Waeselmann et al. (2019).
Transmission 57Fe Mössbauer data for sample RHP7, run at the highest temperature (750 °C), were collected at the University of Salzburg (Austria) in air using an apparatus in a horizontal arrangement (57Fe single-line thin source, constant acceleration mode with symmetric triangular velocity shape, a multichannel analyzer with 1024 channels, regular velocity calibration against metallic Fe). The spectrum was evaluated using the RECOIL program suite (Rancourt and Ping, 1991); data were corrected for thickness effects and then processed using the full static hyperfine interaction Hamiltonian analysis with Lorentzian-shaped doublets.
3.1 Single-crystal X-ray diffraction structure refinement (SREF)
Single-crystal structure refinement data for the riebeckite samples annealed at 0.7 GPa are given in Tables 1 and 2, along with those reported for the untreated riebeckite by Oberti et al. (2018). Previous work shows that the interatomic distance that is most affected by amphibole dehydrogenation is M(1)–M(2) (Oberti et al., 2007); in the samples studied here, the M(1)–M(2) distance does not vary significantly with only a minimal increase of 0.002 Å between 25 and 700 °C (Table 2), which is to be compared to the increase by 0.099 Å observed at atmospheric pressure. Furthermore, the position of the H atom could always be found in different Fourier maps and could also be refined after annealing at 750 °C (Table S1). These observations thus suggest that dehydrogenation does not occur (or occurs only minimally) in riebeckite when annealed up to 750 °C under a pressure of 0.7 GPa, under moderate oxidizing conditions.
However, refinement results indicate the presence of (a small amount of) cation disorder, in line with what was observed by Oberti et al. (2018). When comparing SREF results in terms of total site scattering (measured in electrons per formula unit, Table 1), we observe a slight decrease for the C-type cations (0.8 e−), balanced by a slight increase in the B-type (0.3 e−) and A-type (0.4 e−) cations. These changes, although comparable to the estimated standard deviations (esd) for the refined site scattering, occur progressively from 700 to 750 °C. They are compatible with the presence of a small amount of vacancies at the octahedrally coordinated sites; inspection of the mean cation distances (Table 2) may suggest that these occur mainly at the M(2) and M(3) sites. Oberti et al. (2018) observed the following trend in riebeckite heated between 25 and 700 °C at 1 atm: −5.8 e− for C-type cations, −4.7 e− for B-type cations, and +7.23 e− for the A-type cation. These data were interpreted as a cation migration from the M(4) sites toward the A cavity, which is unusual for amphiboles. This mechanism was also confirmed by the presence of significant electron density at the center of the A cavity in the annealed crystals, which however could not be detected in the crystals analyzed during this work.
In conclusion, the SREF data are coherent with a very limited thermally induced cation migration between different sites under an external pressure of 0.7 GPa, with no signs of transformation of riebeckite into oxo-riebeckite. However, it should be noticed that the results at atmospheric pressure by Oberti et al. (2018) are from heating experiments in air, having oxygen fugacity in log units much higher than those imposed by NNO buffers (ca. −0.68 vs. −17, e.g. Huebner and Sato, 1970). In other words, the oxidation conditions in our annealing experiments differ significantly from those of the in situ heating experiments done by Oberti et al. (2018), which could be the most significant factor accounting for the observed differences. On the other hand, the annealing conditions reported here are closer to the actual conditions experienced by mineral grains in rocks. Therefore, our results clearly show that, at pressures corresponding to a depth of ca. 25 km and temperatures up to 750 °C, there is no oxidation of Fe and, consequently, no H+ loss in riebeckite, unlike what is observed in air at atmospheric pressure (Della Ventura et al., 2018a, 2023).
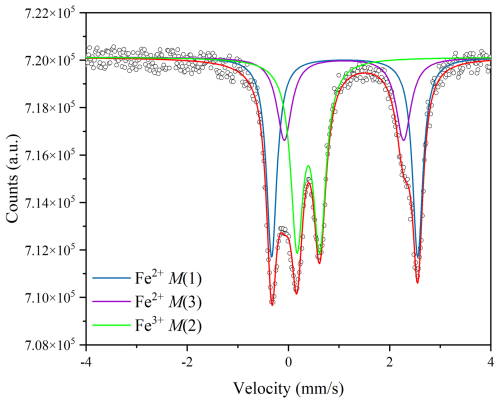
3.2 Mössbauer spectroscopy
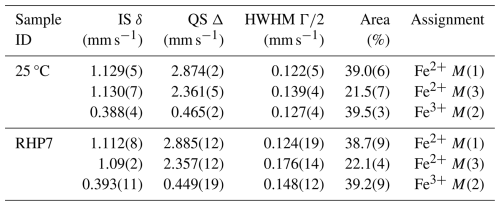
The Mössbauer spectrum of sample RHP7 run at the highest T (750 °C) is given in Fig. 2; it is identical to the spectrum of the pristine riebeckite (Susta et al., 2018; Della Ventura et al., 2023), and its evaluation could be done by using three doublets with no need for additional components. Refined data are given in Table 3, in comparison with those from the untreated sample (from Della Ventura et al., 2023). The inner doublet with quadrupole splitting (Δ, mm s−1) = 0.449(19), the isomer shift (δ, mm s−1) = 0.393(11), and the half width at half maximum (HWHM, mm s−1) = 0.148(12) can be assigned to Fe3+ at M(2); the doublet with Δ=2.357(12), δ=1.09(2) and HWHM = 0.176(14) can be assigned to Fe2+ at M(3); and the doublet with Δ=2.885(12), δ=1.112(18), and HWHM = 0.124(19) can be assigned to Fe2+ at M(1). The relative area fractions for these doublets are , which is very close to the expected area ratio of at for ideal riebeckite. Thus, there is no evidence of significant oxidation of Fe in the sample treated at 750 °C and 0.7 GPa, which is consistent with the SREF results presented above.
3.3 Raman spectroscopy
Raman spectroscopy has been extensively used to study amphiboles (Wang et al., 1988; Della Ventura et al., 1991, 1993; Rinaudo et al., 2004). Collections of spectra from different species have been provided recently by Leissner et al. (2015) and Waeselmann et al. (2019). The latter study in particular showed, based on many spectra from samples spanning a wide range of compositions, that a careful analysis of the Raman data may allow for detailed insights into the crystal-chemistry of the amphibole. A further discussion about the origin of Raman signals and peak assignment to definitive atomic vibrations has been given by Della Ventura et al. (2021a) and (2021b).
Polarized Raman spectra of the studied riebeckite at room temperature and atmospheric pressure have been presented by Susta et al. (2018), whereas the evolution of the scattering as a function of temperature has been discussed by Della Ventura et al. (2018a) and Bernardini et al. (2023). As can be seen in Fig. 3, the relative Raman intensities strongly depend on the scattering geometry, i.e., whether the polarization of the incident light (Ei) is parallel or perpendicular to the polarization of the scattered light (Es) and how the crystal is oriented with respect to Ei. In particular, the OH-stretching signal is strongest in the scattering geometry (c is the corresponding crystallographic axis in C2/m), exhibiting an intense peak at 3619 cm−1 and a weak peak at ∼ 3636 cm−1 (Fig. 3), due to M(1,3)Fe2+Fe2+Fe2+–OH–A□-SiSi and M(1,3)Fe2+Fe2+Mg–OH–A□-SiSi arrangements, respectively (Susta et al., 2018).
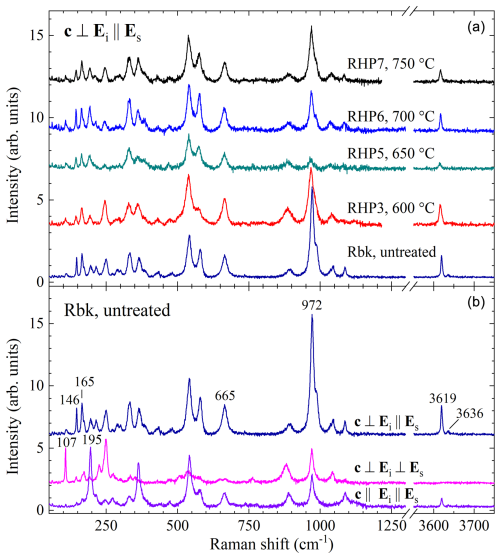
Figure 3(b) Polarized Raman spectra of untreated riebeckite; (a) spectra of the annealed riebeckites collected in the polarization.
Most of the peaks generated by framework vibrations (<1200 cm−1) can be well resolved in parallel polarized spectra () with the exception of the peak at 108 cm−1, assigned to BNa–O modes and possibly external silicate-chain modes (Della Ventura et al., 2021a), that has a maximum intensity in spectra (see Fig. 3). In our previous studies (Della Ventura et al., 2018a; Bernardini et al., 2023), we observed that the M(1,3)O6-related Raman peaks near 146 and 165 cm−1 (best resolved in the spectrum) as well as near 195 cm−1 (strongest in the spectrum) are very sensitive to the oxidation of Fe, and their relative intensity strongly decreases during the Fe2+ → Fe3+ reaction. The SiO4-ring breathing mode near 665 cm−1 as well as the T(1)SiO4 stretching near 971 cm−1, both best resolved in the spectrum, should also be influenced by the oxidation of Fe (Della Ventura et al., 2018a). Moreover, the SiO4-stretching scattering around 970 cm−1 is also sensitive to extended structural defects preceding the collapse of the amphibole structure induced by temperature (Rösche et al., 2022). Thus, to probe for local-scale changes in the structure and crystal chemistry, we measured the annealed samples in all three scattering geometries. As an example, spectra, which contain the largest number of resolved peaks, are compared in Fig. 3. As can be seen, there is no significant variation in the relative intensities of the M(1,3)-O6 peaks (150–200 cm−1), excluding the presence of Fe3+ in the treated samples, as already concluded based on SREF and Mössbauer data. The intensity of the OH-stretching mode relative to the total intensity of the framework-vibration scattering also remains unchanged within the experimental uncertainties, providing robust evidence that there is no significant dehydrogenation of the amphibole up to 750 °C under 0.7 GPa.
3.4 FTIR spectroscopy
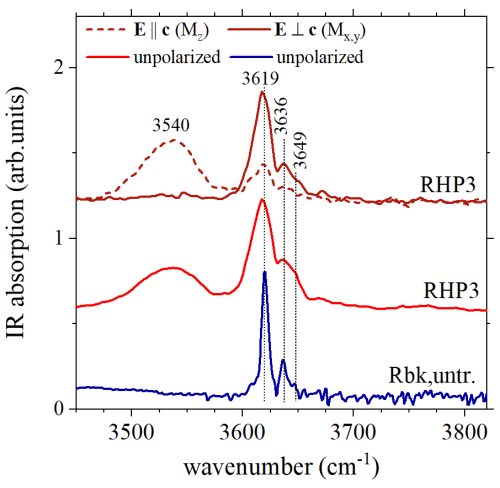
The polarized OH-stretching spectra collected for sample RHP3 (600 °C) are given in Fig. 4. It is well known that when the electric field is perpendicular to the crystallographic axis (E⊥c), the intensity of the OH-stretching mode of amphiboles is maximized (e.g., Skogby and Rossman, 1991), and this is particularly evident in samples having only Si at the T sites and divalent cations at the M(1,3) sites; in this situation, the O–H bond is directed along the a* reciprocal-space axis and thus parallel to the E direction in this setup. The same is true for trioctahedral mica (like phlogopite) when , i.e., parallel to the O–H bond direction (e.g., Scordari et al., 2006). The E⊥c spectrum collected for RHP3 riebeckite (Fig. 4) is similar to the unpolarized spectrum collected at room temperature (e.g., Susta et al., 2018); similar to the Raman patterns, it shows a main relatively sharp peak at 3619 cm−1 which is assigned to local arrangements involving only Fe2+ at the OH-coordinated M(1,3) octahedra, plus weak peaks at ca. 3636 and 3649 cm−1 due to the presence of minor Mg at the same sites (e.g., Susta et al., 2018). Coupled with the Raman data, the main information provided by the FTIR spectrum given in Fig. 4 is that there is no significant loss of OH in the treated samples. A notable increase in the FWHM (full width at half maximum) is apparent in the absorption peaks with respect to those from the untreated crystals; this can be explained based on the increasing structural disorder due to the thermal treatment, as observed via SREF. Interestingly, an additional very broad absorption band centered around 3540 cm−1 appears when (Fig. 4), a feature already observed in unpolarized spectra collected for samples treated at high temperatures by Oberti et al. (2018). The polarization behavior of this broad component indicates that it must be assigned to vibrational modes of O–H dipoles which are differently oriented with respect to the direction of the common hydroxyl groups in the amphibole structure. A possible interpretation for this band will be given below.
The stability of sodic amphiboles has attracted significant attention in the past because of its importance in high-pressure metamorphism and its implications for the release of fluids in subduction zones, which in turn triggers earthquakes and arc volcanism (Cheng et al., 2020; Cheng and Jenkins, 2020; Bang et al., 2021; Manthilake et al., 2021). Most experiments have been focused on glaucophane, nominally A□BNa2C(Mg3Al2)TSi8O22(OH)2, although rock-forming minerals typically have intermediate compositions in the glaucophane–magnesio-arfvedsonite–magnesio-riebeckite system and their iron counterparts (Miyashiro and Banno, 1958; Deer et al., 1997). Conflicting results have been reported by different authors, primarily due to (a) deviations of the synthesized amphiboles from the nominal glaucophane stoichiometry (see Gillet et al., 1989; Tropper et al., 2000) and (b) the persistent occurrence of a sheet silicate close to vermiculite in the run assemblage (Jenkins and Corona, 2006). Earlier studies (e.g., Ernst, 1961; 1963b; Carman and Gilbert, 1983) reported the existence of two polymorphs, glaucophane I and glaucophane II (Ernst, 1963b), characterized by notably different stability fields. Maresch (1973) has later revised the phase relations in the Na2O–MgO–SiO2–H2O system and demonstrated that the two polymorphs were two different amphibole species with a high degree of substitutional disorder. The low-pressure polymorph (previously named glaucophane I) was shown to be in fact compositionally similar to “magnesio-richterite” (root name 8 after Hawthorne et al., 2012), nominally ANaB(NaMg)CMg5TSi8O22(OH)2. Jenkins and Corona (2006) investigated the role of water on the synthesis of glaucophane and showed that the optimal conditions to obtain in-composition products during high-temperature and/or high-pressure (HT/HP) experiments was using 4 wt %–5 wt % H2O for long durations (400 h) combined with multiple treatments and intermediate grindings. According to the experiments of Bang et al. (2021), the maximum depth of glaucophane stability increases for decreasing thermal gradients of the subduction system. Along cold geotherms, glaucophane may remain stable down to 240 km, whereas in warm subduction zones it breaks down and dehydrates at much shallower depths (ca. 40 km). This difference has major implications for phenomena such as rock conductivity, seismicity, and volcanism (see also Bernardini et al., 2023). However, it is important to note that the stability data obtained from experiments done under water-saturated conditions have been recently questioned by Putak-Juricek and Keppler (2023) based on the consideration that most of the mantle may contain only traces of water. They thus conducted new experiments at very low H2O fugacity, and the results showed that decreasing water activity significantly shifts the stability field of the amphibole to lower pressures while expanding it to higher temperatures.
The stability of Fe-rich compositions is less studied. Hoffmann (1972) examined the phase relations of ferro-glaucophane, ideally A□BNa2C(FeAl2)TSi8O22(OH)2, over the temperature range 250–500 °C and pressures up to 0.5 GPa under varying . He observed that at relatively low (using wüstite-magnetite (WM) and fayalite-magnetite-quartz (FMQ) buffers), ferro-glaucophane breaks down above 350–360 °C, with its stability further reduced under oxidizing conditions. Magnesio-riebeckite, ideally A□BNa2C(Mg3Fe)TSi8O22(OH)2, and riebeckite were first synthesized by Ernst (1960, 1962) under different buffering conditions. Magnesio-riebeckite was found to remain stable up to 950 °C at fluid pressures above 0.2 GPa when was controlled by the hematite-magnetite (HM) buffer. However, its stability decreased by ca. 100 °C under reducing conditions. In contrast, riebeckite was found to be stable at much lower temperatures (around 500 °C) under the HM buffer, with decomposition products fayalite + magnetite + quartz + vapor (Ernst, 1962). Della Ventura et al. (2005) synthesized magnesio-riebeckite at constant T (700 °C) and P (0.4 GPa) under varying conditions, and they observed that the composition of the amphibole linearly shifted toward magnesio-arfvedsonite (ANaBNa2C(Mg4Fe3+)TSi8O22(OH)2) as increased, as observed in natural occurrences (e.g., Deer et al., 1997). This conclusion aligns with the findings of Della Ventura et al. (2023) and Oberti et al. (2018) as well as with the results of the present study, which shows an increasing structural disorder with small but detectable migration of Na toward the A site during thermal treatment under oxidizing conditions.
The above discussion emphasizes that, while the geological stability of sodium amphiboles has received significant attention, the effect of pressure on the crystal-chemical processes driving Reaction (1) has never been thoroughly investigated.
The oxidation behavior of riebeckite as a function of temperature at ambient pressure has been extensively studied during 1960–1970 because of the technological interest in its fibrous asbestos variety, crocidolite (e.g., Addison et al., 1962a, 1962b; Ernst and Wai, 1970, among others). Detailed studies done during the last decade – and comprehensively reviewed by Della Ventura et al. (2024b) – have shown that iron oxidation in amphiboles is reversible across a relatively wide thermal range. This reversibility persists until the kinetic energy of delocalized electrons and H+ ions becomes high enough to allow for their ejection from the structure. The entire process involves a series of structural rearrangements as a response to the local charge imbalances generated by Reaction (1). A complete characterization of these rearrangements requires the integration of diffraction and spectroscopic methods.
From a geophysical perspective, the major issue associated with Reaction (1) is the dehydrogenation accompanying iron oxidation, because this process has direct implications for water supply at depth in subduction regimes (Bang et al., 2021). As discussed by Della Ventura et al. (2018a, 2023), dehydrogenation is, however, strongly dependent on the availability of external oxygen. Another critical issue is the development of charge carriers (electrons and H+ ions), resulting from Reaction (1), which has significant implications for the geophysical structure of the lower-crust–mantle system. As recently discussed by Manthilake et al. (2021), the direct relationship among temperature, Fe redox process, dehydration, and electrical conductivity can be used to benchmark the thermal structure of the descending slab (information that is critical for modeling the geological processes at convergent margins) based on electrical measurements. This observation stresses the importance of understanding the crystal-chemical consequences of Reaction (1) as a function of P, T, and .
Our data definitively show that pressure has a significant effect on oxidation of Fe in riebeckite, shifting it toward higher temperatures. In particular, single-crystal structure refinements show that there are no major modifications in the site occupancies up to 750 °C when annealing the crystals at 0.7 GPa. Analysis of the SREF results (Table 1) shows that there is almost no oxidation of Fe and no dehydrogenation as well as no change in the M(1,3)FeFe3+ site occupancy. This conclusion is in full agreement with Mössbauer results obtained for the crystal annealed at the highest T (750 °C), as well as with OH-stretching FTIR and Raman spectroscopy. Interestingly, electron densities in the sample annealed at 1 atm clearly indicate a slight but significant depletion of B-type and C-type cations, coupled with an increase in A-type cations. This implies the creation of vacancies at the M(3) and M(4) sites (Oberti et al., 2018) and correlates with the appearance of a broad band at 3540 cm−1 in the FTIR spectrum (Fig. 4), whose wavenumber is notably close to values known for local dioctahedral configurations in mica (e.g., Robert et al., 1989). This broad band is present in the FTIR spectra collected on the annealed samples, with maximum intensity when (Fig. 4), indicating that the O–H bonds absorbing the IR beam are preferably oriented along [001], rather than perpendicular to the MO6 strips. Such an orientation is similar to that of the hydroxyl groups in dioctahedral mica, where the O–H bonds are tilted toward the octahedral layer to compensate for missing charges caused by octahedral vacancies (“vacancy bands”; see Della Ventura et al., 2024c). For instance, in muscovite, the O–H bond is tilted 16° away from the (001) crystallographic plane (Vedder and McDonald, 1963). The above discussion thus provides additional support to the inference that the broad band at 3540 cm−1 in annealed crystals is related to partially vacant octahedrally coordinated sites within their structure.
The absence of irreversible oxidation of Fe and the increase in structural disorder in riebeckites annealed at 0.7 GPa is further confirmed by Raman spectroscopy (Fig. 3). In particular, the wavenumber of the SiO4 ring mode (near 665 cm−1) of the annealed samples does not show any increase with respect to untreated riebeckite (Fig. 5a), which confirms that the valence state of Fe remains the same after annealing under 0.7 GPa; within uncertainties, the SiO4-ring mode instead shows a subtle tendency to shift to lower phonon energies (i.e., wavenumbers). The SiO4-stretching mode at ca. 970 cm−1 has an even more pronounced decrease in phonon energy (Fig.5b), implying a development of structural defects when considering that connectivity defects loosen the structure and, hence, weaken the interatomic interactions. The same conclusion can be deduced from the wavenumber of the phonon mode at 108 cm−1 (Fig. 5c), which involves external silicate-chain vibrations (Waeselmann et al., 2019). Occurrence of structural disorder should also affect the phonon FWHMs, but the variation in FWHM for most of the Raman peaks is insufficient to draw any solid conclusions. Yet, a few MO6-related peaks, e.g., that at 165 cm−1 (see Fig. 5d), steadily broaden with the increase in the annealing temperature.
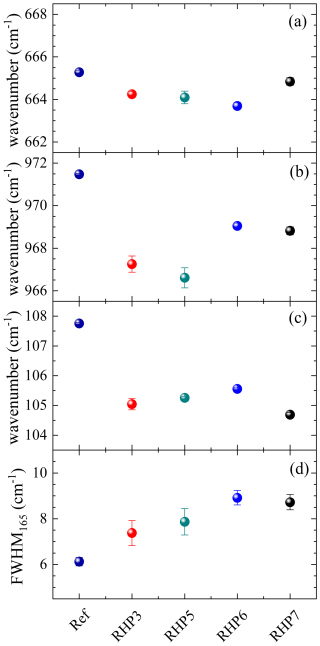
Figure 5Evolution of the wavenumber of (a) the SiO4 ring mode, (b) the SiO4-stretching mode, (c) the BNa–O/external silicate chain mode, and (d) the FWHM of the M(1,3)O6-related phonon mode at ca. 165 cm−1 as for riebeckites annealed at 0.7 GPa and increasing T (for the sample codes, see the main text). Ref = untreated sample. For the mode assignments, see Della Ventura et al. (2018a) and Waeselmann et al. (2019).
Summarizing, all crystal-chemical data converge in concluding that when riebeckite is annealed up to 750 °C at 0.7 GPa (corresponding to approximately 25 km depth and under redox conditions close to the NNO buffer) there is no dehydrogenation and, consequently, no oxidation of Fe in the sample; SREF data show complete Fe3+ ordering at M(2), a feature that, based on the work of Della Ventura et al. (2023), provides additional robust proof for the oxidizing conditions during the thermal treatment.
The H2O transport into the deep Earth occurs through hydrous minerals in subducting crust, whose stability range is primarily controlled by the P–T redox conditions of the subducting system (e.g., Peacock and Wang, 1996; Kimura and Nakajima, 2014; Manthilake et al., 2016). The release of fluids during metamorphism controls seismicity, partial melting of lithospheric crust, and volcanism. It is therefore essential to investigate the stability of hydrous minerals as a function of varying physical conditions and great efforts have been spent in this direction during the last decade; this work represents a further piece of this puzzle.
In previous papers, we reported on our studies on the thermal behavior of riebeckite, monitoring the oxidation of Fe and the consequent dehydration and development of electrical conductivity at ambient pressure (Oberti et al., 2018; Bernardini et al., 2023; Della Ventura et al., 2024a). We compared the experimentally derived temperatures for the development of charge carriers (hopping electrons and delocalized H+) with the P–T conditions inferred (Peacock and Wang, 1996) in warm and cold subduction regimes and observed significant agreement of our data with the electrical structures reconstructed from magnetotelluric data (Kasaya et al., 2005). Here we provide a further step toward elucidating the role of redox processes in hydrous silicates on the conductivity anomalies of the crust, by simulating conditions closer to those of lithospheric rocks. We show that with increasing pressure to 0.7 GPa, as well as NNO oxygen fugacity, the stability of riebeckite is significantly extended to ca. 150 °C, with no irreversible oxidation of Fe and H+ loss. To the best of our knowledge, the only work done in situ at HP via Mössbauer spectroscopy on synthetic magnesio-riebeckite shows a significant reduction of octahedrally coordinated Fe in amphiboles for P>2 GPa (Burns et al., 1972), an unexpected feature that needs additional verification. In fact, Fe reduction at in a silicate-like riebeckite implies the diffusion of H+ ions from the exterior to maintain local charge equilibria and structure stability. It is also worth noting that Fe reduction when treating pargasite up to 1100 °C at 0.1 GPa had already been observed by Popp et al. (2006). If confirmed, this process would have interesting implications in both geophysics and in material science.
Using resonance Raman spectroscopy (Mihailova et al., 2021, 2022), Bernardini et al. (2023) demonstrated that at atmospheric pressure riebeckite develops anisotropic polaron hopping (and thus electric conduction) for T>227 °C, with further conduction occurring for T>377 °C due to additional charge contribution from delocalized H+ ions. However, the role of pressure in this process remains poorly understood. According to Goddat et al. (1999), small-polaron conduction increases with pressure due to its negative activation volume. For riebeckite, in particular, the conductivity increases by approximately 1 order of magnitude for a pressure increase of 2 GPa in the temperature range of 200 to 600 °C (Parkomenko, 1982). The experimental data described here demonstrate that the crystal-chemical rearrangement associated with irreversible Fe2+ to Fe3+ oxidation is shifted to higher temperatures when P is increased to 0.7 GPa. This suggests that (1) the electric conduction due to electron hopping in amphibole-bearing subducted rocks may occur at greater depths in descending slabs, and (2) pressure significantly increases the depth at which water is released, with important implications for global water cycling and associated geological processes. Although is considered to have limited relevance for the bulk stability of the amphiboles at mantle conditions (e.g., Mandler and Grove, 2016), the work of Della Ventura et al. (2023) demonstrates that, in the presence of external oxygen, water release occurs at significantly lower temperatures compared to reducing conditions; the results presented here, however, suggest that water release is inhibited by pressure.
As a final note, our work shows that accurate crystal-chemical data, obtained through the integration of XRD and spectroscopic measurements done under varying environmental conditions (i.e., T, P, ), are critical for understanding the relation between mineral stability and geophysical phenomena.
All raw data can be provided by the corresponding authors upon request.
The supplement related to this article is available online at https://doi.org/10.5194/ejm-37-535-2025-supplement.
GDV and FR planned the experiments; GDV, FR, and VM performed the experiments; GDV, RO, MB, GJR, BM, and SB performed the measurements; GDV, RO, MB, GJR, BM, and SB analyzed the data; GDV wrote the first draft; all authors reviewed and edited the manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Celebrating the outstanding contribution of Paola Bonazzi to mineralogy”. It is not associated with a conference.
Thanks are due to referees F. C. Hawthorne (Winnipeg, Canada) and G. Iezzi (Chieti, Italy) for helping to clarify some aspects of our work.
This paper is dedicated to Paola Bonazzi, a leading crystallographer and mineralogist at the University of Florence and, above all, a very gifted and nice person as well as a dear friend for many years.
Financial support was provided by the Federal Ministry of Education and Research (BMBF) and the Free and Hanseatic City of Hamburg under the Excellence Strategy of the Federal Government and the Länder, by Deutsche Forschungsgemeinschaft (grant no. MI 1127/13-1), and by the grant to Department of Science, Roma Tre University (MIUR-Italy Dipartimenti di Eccellenza, ARTICOLO 1, COMMI 314-337 LEGGE 232/2016).
This paper was edited by Luca Bindi and reviewed by Frank Hawthorne and Gianluca Iezzi.
Addison, C. C., Addison, W. E., Neal, G. H., and Sharp J. H.: Amphiboles. Part I. The oxidation of crocidolite, J. Chem. Soc., 1468–1471, 1962a.
Addison, W. E., Neal, G. H., and Sharp, J. H.: Amphiboles. Part II. The kinetics of oxidation of crocidolite, J. Chem. Soc., 1472–1475, 1962b.
Bang, Y., Hwang, H., Kim, T., et al.: The stability of subducted glaucophane with the Earth's secular cooling, Nat. Commun., 12, 1496, https://doi.org/10.1038/s41467-021-21746-8, 2021.
Bernardini, S., Della Ventura, G., Schluter, J., and Mihailova, B.: Thermally activated electron hopping in Fe-rich amphiboles: implications for the high-conductivity anomalies in subduction zones, Geochemistry, 83, 125942, https://doi.org/10.1016/j.chemer.2022.125942, 2023.
Bernardini, S., Della Ventura, G., Schlüter, J., Hawthorne, F. C., and Mihailova, B.: The effect of A-site cations on the charge-carrier mobility in Fe-bearing amphiboles, Am. Mineral., 109, 1545–1553, https://doi.org/10.2138/am-2023-9138, 2024, 2024.
Blessing, R. H., Coppens, P., and Becker, P.: Computer analysis of step scanned X-ray data, J. Applied Cryst., 7, 488–492, 1974.
Burns, R. G., Tossell, J. A., and Vaughan, D. J.: Pressure-induced reduction of a ferric amphibole, Nature, 240, 33–35, 1972.
Cannillo, E., Germani, G., and Mazzi, F.: New crystallographic software for Philips PW11000 single crystal diffractometer, CNR Centro di Studio per la Cristallografia, Internal Report 2, 1983.
Carman, J. H. and Gilbert, M. C.: Experimental studies on glaucophane stability, Am. J. Sci., 283-A, 414–437, 1983.
Cheng, N. and Jenkins, D. M.: Experimental study of metamorphic reactions and dehydration processes at the blueschist–eclogite transition during warm subduction, J. Met. Geol., 39, 39–56, https://doi.org/10.1111/jmg.12560, 2020.
Cheng, N., Jenkins, D. M., and Huang F.: Dehydration of glaucophane in the system Na2O–MgO–Al2O3–SiO2–H2O and the effects of NaCl-, CO2- and silicate-bearing aqueous fluids, J. Petrol., 60, 2369–2386, 2020.
Deer, W. A., Howie, R. A., and Zussman, J.: Rock-forming Minerals, vol 2B, Double-Chain Silicates, 2nd edn., 764 p., Geological Society, London, ISBN 1-897799-77-2, 1997.
Della Ventura, G., Robert, J.-L., and Bény, J.-M.: Tetrahedrally coordinated Ti4+ in synthetic Ti-rich potassic richterite: Evidence from XRD, FTIR and Raman studies, Am. Mineral., 76, 1134–1140, 1991.
Della Ventura, G., Robert, J.-L., Bény, J.-M., Raudsepp, M., and Hawthorne, F. C.: The OH-F substitution in Ti-rich potassium-richterites: Rietveld structure refinement and FTIR and microRaman spectroscopic studies of synthetic amphiboles in the system K2O–Na2O–CaO–MgO–SiO2–TiO2–H2O–HF, Am. Mineral., 78, 980–987, 1993.
Della Ventura, G., Iezzi, G., Redhammer, G. J., Hawthorne, F. C., Scaillet, B., and Novembre, D.: Synthesis and crystal-chemistry of alkali amphiboles in the system Na2O–MgO–FeO–Fe2O3–SiO2–H2O as a function of , Am. Mineral., 90, 1375–1383, 2005.
Della Ventura, G., Mihailova, B., Susta, U., Cestelli-Guidi, M., Marcelli, A., Schlüter, J., and Oberti, R.: The dynamics of Fe oxidation in riebeckite: A model for amphiboles, Am. Mineral., 103, 1103–1111, 2018a.
Della Ventura, G., Galdenzi, F., Cibin, G., Oberti, R., Xu, W., Macis, S., and Marcelli, A.: Iron oxidation dynamics vs. temperature of synthetic potassic-ferro-richterite: a XANES investigation, Phys. Chem. Chem. Phys., 20, 21764–21771, 2018b.
Della Ventura, G., Mihailova, B., and Hawthorne, F. C.: Raman and FTIR spectroscopy of synthetic amphiboles: II. Divalent (Mg-Co) substitutions at the octahedral sites, Can. Mineral., 59, 43–57, 2021a.
Della Ventura, G., Hawthorne, F. C., Mihailova, B., and Sodo, A.: Raman and FTIR spectroscopy of synthetic amphiboles: I. The OH librational bands and the determination of the OH–F content of richterites via Raman spectroscopy, Can. Mineral., 59, 31–41, 2021b.
Della Ventura, G., Radica, F., Galdenzi, F., Susta, U., Cinque, G., Mihailova, B., and Marcelli, A.: Kinetics of hydrogen diffusion in riebeckite, Na2FeFeSi8O22(OH)2: an HT-FTIR study, Am. Mineral., 107, 754–764, 2022.
Della Ventura, G., Redhammer, G. J., Galdenzi, F., Ventruti, G., Susta, U., Oberti, R., Radica, F., and Marcelli, A.: Oxidation or cation re-arrangement? Distinct behavior of riebeckite at high temperature, Am. Mineral., 108, 59–69, 2023.
Della Ventura, G., Galdenzi, F., Marcelli, A., Cibin, G., Oberti, R., Hawthorne, F. C., Bernardini, S., and Mihailova, B.: In situ simultaneous Fe K-edge XAS spectroscopy and resistivity measurements of riebeckite: implications for anomalous electrical conductivity in subduction zones, Geochemistry, 84, 126037, https://doi.org/10.1016/j.chemer.2023.126037, 2024a.
Della Ventura, G., Bernardini, S., Redhammer, G. J., Galdenzi, F., Radica, F., Marcelli, A., Hawthorne, F. C., Oberti, R., and Mihailova, B.: The oxidation of iron in amphiboles at high temperatures: a review and implications for large-scale Earth processes, Rend. Fis. Acc. Lincei, 35, 893–906, https://doi.org/10.1007/s12210-024-01280-7, 2024b.
Della Ventura, G., El Moutouakkil, N., Boukili, B., Bernardini, S., Sodo, A., Cestelli-Guidi, M., Holtz, F., and Lucci, F.: Tracking the Ti4+ substitution in phlogopite by spectroscopic imaging: a tool for unrevelling the growth of micas at high pressute/temperature, Geosci. Front., 15, 101777, https://doi.org/10.1016/j.gsf.2024.101777, 2024c.
Ernst, W. G.: The stability relations of magnesioriebeckite, Geochim. Cosmochim. Ac., 19, 10–40, 1960.
Ernst, W. G.: Stability relations of glaucophane, Am. J. Sci., 259, 735–765, 1961.
Ernst, W. G.: Synthesis, stability relations and occurrence of riebeckite and riebeckite-arfvedsonite solid-solutions, J. Geol., 70, 689–736, 1962.
Ernst, W. G.: Petrogenesis of glaucophane schists, J. Petrol., 4, 1–30, 1963a.
Ernst, W. G.: Polymorphism in alkali amphiboles, Am. Mineral., 48, 241–260, 1963b.
Ernst, W. G. and Wai, M.: Mössbauer, infrared, X-ray and optical study of cation ordering and dehydrogenation in natural and heat-treated sodic amphiboles, Am. Mineral., 55, 1226–1258, 1970.
Gillet, P., Reynard, B., and Tequi, C.: Thermodynamic properties of glaucophane. New data from calorimetric and spectroscopic measurements, Phys. Chem. Miner., 16, 659–667, 1989.
Goddat, A., Peyronneau, J., and Poirier, J. P.: Dependence on pressure of conduction by hopping of small polarons in minerals of the Earth's lower mantle, Phys. Chem. Miner., 27, 81–87, 1999.
Hawthorne, F. C. and Oberti, R.: Amphiboles: Crystal-chemistry, in: Amphiboles: Crystal Chemistry, Occurrence and Health Issues, edited by: Hawthorne, F. C., Oberti, R., Della Ventura, G., and Mottana, A., Rev. Mineral, Geochemistry, 67, 1–54, https://doi.org/10.2138/rmg.2007.67.1, 2007.
Hawthorne, F. C, Ungaretti, L., and Oberti, R.: Site populations in minerals: terminology and presentation of results of crystal-structure refinement, Can. Mineral., 33, 907–911, 1995.
Hawthorne, F. C., Oberti, R., Harlow, G. E., Maresch, W. V., Martin, R. F., Schumacher, J. C., and Welch, M. D.: Nomenclature of the amphibole supergroup, Am. Mineral., 97, 2031–2048, 2012.
Hoffmann, C.: Natural and synthetic ferroglaucophane, Contr. Mineral. Petrol., 34, 135–149, 1972.
Howe, H., Pawley, A. R., and Welch, M. D.: Sodium amphibole in the post-glaucophane high-pressure domain: the role of eckermannite, Am. Mineral., 103, 989–992, 2018.
Hu, H., Dai, L., Li, H., Sun, W., and Li, B.: Effect of dehydrogenation on the electrical conductivity of Fe-bearing amphibole: Implications for high conductivity anomalies in subduction zones and continental crust, Earth Planet. Sc. Lett., 498, 27–37, https://doi.org/10.1016/j.epsl.2018.06.003, 2018.
Huebner J. S. and Sato, M.: The oxygen fugacity – temperature relationships of manganese oxide and nickel oxide buffers, Am. Mineral., 55, 934, 1970.
Kasaya, T., Goto, T., Mikada, H., Baba, K., Suyehiro, K., and Utada, H.: Resistivity image of the Philippine Sea Plate around the 1944 Tonankai earthquake zone deduced by Marine and Land 579 MT surveys, Earth Planet. Space, 57, 209–213, 2005.
Kawamoto, T. and Hirose, K.: Au-Pd sample containers for melting experiments on iron and water bearing systems, Eur. J. Mineral., 6, 381–385, 1994.
Kimura, J.-I. and Nakajima, J.: Behaviour of subducted water and its role in magma genesis in the NE Japan arc: A combined geophysical and geochemical approach, Geochim. Cosmochim. Ac., 143, 165–188, 2014.
Kushiro, I.: Partial melting of mantle wedge and evolution of island arc crust, J. Geophys. Res., 95, 15929–15939, 1990.
Jenkins, D. M. and Corona, J.-C.: The role of water in the synthesis of glaucophane, Am. Mineral., 91, 1055–1068, 2006.
Lehmann, M. S. and Larsen, F. K.: A method for location of the peaks in step-scan-measured Bragg reflections, Acta Cryst., A30, 580–584, 1974.
Leissner, L., Schlüter, J., Horn, I., and Mihailova, B.: Exploring the potential of Raman spectroscopy for crystallochemical analyses of complex hydrous silicates: I. Amphiboles, Am. Mineral., 100, 2682–2694, 2015.
Mandler, B. E. and Grove, T. L.: Controls on the stability and composition of amphibole in the Earth's mantle, Contrib. Mineral. Petrol., 171, 68, https://doi.org/10.1007/s00410-016-1281-5, 2016.
Manthilake, G., Bolfan-Casanova, N., Novella, D., Mookherjee, M., and Andrault, D.: Dehydration of chlorite explains anomalously high electrical conductivity in the mantle wedges. Sci. Adv., 2, e1501631, https://doi.org/10.1126/sciadv.1501631, 2016.
Manthilake, G., Peng, Y., Koga, K. T., and Mookherjee, M.: Tracking slab surface temperatures with electrical conductivity of glaucophane, Sci. Rep., 11, 18014, https://doi.org/10.1038/s41598-021-97317-0, 2021.
Maresch, W. V.: New data on the synthesis and stability relations of glaucophane, Earth Planet. Sc. Lett., 20, 385–390, 1973.
Mihailova, B., Della Ventura, G., Waeselmann, N., Wei, Xu, Schlüter, J., Galdenzi, F., Marcelli, A., Redhammer, G. J., Boiocchi, M., and Oberti, R.: Atomistic insight into lithospheric conductivity revealed by phonon-electron excitations in hydrous iron-bearing silicates, Comm. Mat., 2, 57, https://doi.org/10.1038/s43246-021-00161-y, 2021.
Mihailova, B., Della Ventura, G., Waeselmann, N., Bernardini, S., Wei, X., and Marcelli, A.: Polarons in rock-forming minerals: Physical implications, Condensed Matter, 7, 68, https://doi.org/10.3390/condmat7040068, 2022.
Miyashiro, A. and Banno, S.: Nature of glaucophanitic metamorphism, Am. J. Sci., 256, 97–110, 1958.
Momma, K. and Izumi, F.: VESTA: a three-dimensional visualization system for electronic and structural analysis, J. Appl. Crystallogr., 41, 653–658, 2008.
North, A. C. T., Phillips, D. C., and Mathews, F. S.: A semi-empirical method of absorption correction, Acta Cryst., A24, 351–359, 1968.
Oberti, R., Hawthorne, F. C., Cannillo, E., and Cámara, F.: Long-range order in amphiboles, in: Amphiboles: Crystal Chemistry, Occurrence and Health Issues, edited by: Hawthorne, F. C., Oberti, R., Della Ventura, G., and Mottana, A., Rev. Mineral, Geochemistry, 67, 125–172, https://doi.org/10.2138/rmg.2007.67.4, 2007.
Oberti, R., Boiocchi, M., Zema, M., and Della Ventura, G.: Synthetic potassic-ferro-richterite: 1. Composition, crystal structure refinement and HT behavior by in operando single-crystal X-ray diffraction, Can. Mineral. 54, 353–369, 2016.
Oberti, R., Della Ventura, G., Boiocchi, M., Zanetti, A., and Hawthorne, F. C.: New data on the crystal-chemistry of oxo-mangani-leakeite and mangano-mangani-ungarettiite from the Hoskins mine and their impossible solid-solution – An XRD and FTIR study, Min. Mag., 81, 707–722, 2017.
Oberti, R., Boiocchi, M., Zema, M., Hawthorne, F. C., Redhammer, G. J., Susta, U., and Della Ventura, G.: The high-temperature behaviour of riebeckite: expansivity, deprotonation, selective Fe oxidation and a novel cation disorder scheme for amphiboles, Eur. J. Mineral., 30, 437–449, 2018.
Parkomenko, E. I.: Electrical resistivity of minerals and rocks at high temperature and pressure, Rev. Geophys. Space Phys., 20, 193–218, 1982.
Peacock, S. M. and Wang, K.: Seismic consequences of warm versus cool subduction metamorphism: examples from Southwest and Northeast Japan, Science, 286, 937–939, 1996.
Phillips, M. W., Popp R. K., and Clowe, C. A.: Structural adjustments accompanying oxidation-dehydrogenation in amphiboles, Am. Mineral., 73, 500–506, 1988.
Popp, R. K., Hibbert, H. H., and Lambi, W. M.: Oxy-amphibole equilibria in Ti-bearing calcic amphiboles: Experimental investigation and petrologic implications for mantle-derived amphiboles, Am. Mineral., 91, 54–66, 2006.
Putak-Juricek, M. and Keppler, H.: Amphibole stability, water storage in the mantle, and the nature of the lithosphere-asthenosphere boundary, Earth Planet. Sc. Lett., 608, 118082, https://doi.org/10.1016/j.epsl.2023.118082, 2023.
Rancourt, D. G. and Ping, J. Y.: Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy, Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 58, 85–97, 1991.
Rinaudo, C., Belluso, E., and Gastaldi, D.: Assessment of the use of Raman spectroscopy for the determination of amphibole asbestos, Min. Mag., 68, 455–465, 2004.
Robert, J.-L., Beny, J.-M., Beny, C., and Volfinger, M.: Characterization of lepidolites by Raman and infrared spectrometries. I. Relationship between OH-stretching wavenumbers and composition, Can. Mineral., 27, 225–235, 1989.
Rösche, C., Waeselmann, N., Petrova, N., Malcherek, T., Schlüter, J., and Mihailova, B.: Oxidation processes and thermal stability of actinolite, Phys. Chem. Mineral., 49, 1–17, https://doi.org/10.1007/s00269-022-01223-4, 2022.
Schmidt, M. W. and Poli, S.: Experimentally based water budgets for dehydrating slabs and consequences for arc magma generation, Earth Planet. Sc. Lett., 163, 361–379, 1998.
Scordari, F., Ventruti, G., Sabato, A., Bellatreccia, F., Della Ventura, G., and Pedrazzi, G.: Ti-rich phlogopite from Mt. Vulture (Potenza, Italy): a multianalytical approach, substitutional mechanisms and the orientation of the OH-dipoles, Eur. J. Mineral., 18, 379–391, 2006.
Skogby, H. and Rossman, G. R.: The intensity of amphiboles OH bands in the infrared absorption spectrum, Phys. Chem. Mineral., 18, 64–68, 1991.
Sisson, V. B., Ertan, I. E., and Avé Lallemant, H. G.: High pressure (∼ 2000 MPa) kyanite- and glaucophane-bearing pelitic schists and eclogite from Cordillera de la Costa belt, Venezuela, J. Petrol., 38, 65–83, 1997.
Susta, U., Della Ventura, G., Hawthorne, F. C., Milahova, B., and Oberti, R.: The crystal-chemistry of riebeckite, ideally Na2FeFeSi8O22(OH)2: a multi-technique study, Min. Mag., 84, 837–852, 2018.
Tropper, P., Manning, C. E., Essene, E. J., and Kao, L. S.: The compositional variation of synthetic sodic amphiboles at high and ultra-high pressures, Contrib. Mineral. Petrol., 139, 146–162, 2000.
Vedder, W. and McDonald, R. S.: Vibrations of the OH ions in muscovite, J. Chem. Phys., 38, 1583–1590, 1963.
Waeselmann, N., Schlüter, J., Malcherek, T., Della Ventura, G., Oberti, R., and Mihailova, B.: Nondestructive determination of the amphibole crystal-chemistry formulae by Raman spectroscopy: One step closer, J. Raman Spectr., 2019, 1530–1548, https://doi.org/10.1002/jrs.5626, 2019.
Wang, A., Dhamelincourt, P., and Turrell, G.: Raman microspectroscopic study of the cation distribution in amphiboles, Appl. Spec., 42, 1441–1450, 1988.
Wang, D., Guo, Y., Yu, Y., and Karato, S.: Electrical conductivity of amphibole-bearing rocks: influence of dehydration, Contrib. Mineral. Petrol., 164, 17–25, 2012.