the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Karlseifertite, Pb(Ga2Ge)(AsO4)2(OH)6, a new dussertite-group mineral, from Tsumeb, Namibia
Joy Désor
Karlseifertite (IMA 2024-007, International Mineralogical Association), Pb(Ga2Ge)(AsO4)2(OH)6, is a new member of the dussertite group, from Tsumeb, Namibia. It is a secondary oxidation-zone mineral found on fracture surfaces in germanite–chalcocite ore. Karlseifertite occurs in rosettes of thin, yellow, hexagonal plates up to about 0.2 mm in diameter and usually less than 0.01 mm thick. The mineral has a pale-yellow streak, subadamantine lustre, Mohs hardness of ∼ 4, brittle tenacity, irregular fracture, perfect cleavage on {001} and a calculated density of 4.993 g cm−3. Optically, karlseifertite crystals are uniaxial (+), with ω=1.890(5) and ε=1.894(calc) (white light). The empirical formula from electron probe microanalyses is Pb(GaGeFeAl)Σ2.97[(AsSW)Σ1.00O4]2(OH0.99)6. Karlseifertite is trigonal with space group and unit-cell parameters a=7.2814(7), c=17.1077(12) Å, V=785.50(15) Å3 and Z=3. The mineral has an alunite-supergroup structure (R1=0.0591 for 248 reflections with I>2σI).
- Article
(3774 KB) - Full-text XML
-
Supplement
(189 KB) - BibTeX
- EndNote
The minerals of the alunite supergroup (Bayliss et al., 2010) have the general formula , where (as far as dominant ions are concerned) D is usually a relatively large monovalent, divalent or trivalent cation; G is usually a relatively small trivalent cation (commonly Al or Fe3+); T is a pentavalent or hexavalent cation (generally restricted to P5+, As5+ and/or S6+); X is O or OH; and X′ is usually OH−. In the wide range of minerals included in the alunite supergroup, electroneutrality is maintained by coupled substitutions among the D, G, T and X sites in the structure. The groups within the alunite supergroup (dictated by the T cations) include the alunite group (T = S6+), plumbogummite group (T = P5+), dussertite group (T = As5+) and beudantite group (T = P5+/As and S). The presence of P or As and S in the ideal formulas of members of the beudantite group is an example of a single-site heterovalent substitution resulting in valency-imposed double site occupancy (Hatert and Burke, 2008).
Single-site heterovalent substitutions resulting in valency-imposed double site occupancy only rarely occur in the D or G sites. Heretofore, only three alunite-supergroup mineral species have been described based on valency-imposed double site occupancy in the G site: beaverite–(Cu), Pb(FeCu2+)(SO4)2(OH)6; beaverite–(Zn), Pb(FeZn)(SO4)2(OH)6; and osarizawaite, Pb(Al2Cu2+)(SO4)2(OH)6. All are in the alunite group. Karlseifertite, the new species described herein, with the ideal formula Pb(GaGe4+)(AsO4)2(OH)6, is the first member of the dussertite group to be described based upon valency-imposed double site occupancy in the G site. It is also the first member of the alunite supergroup containing essential Ge.
The name karlseifertite honours the German mining engineer Karl Markus Seifert (1911–2000). Mr Seifert's professional career spanned nearly 50 years, much of which was spent as an engineer and prospector for mining companies in Namibia (South West Africa for most of that period). From 1963 to 1967, he was mining engineer for Tsumeb Corporation Ltd. (TCL), which operated the Tsumeb mine (Tsumcorp mine). Karl Seifert was well known for his expertise in geology and mineralogy, particularly with respect to mineral exploration. He also had a passion for mineral collecting and assembled an extensive collection through his many diverse mining pursuits. The mineral named in his honour was found on specimens that he collected as mining engineer at Tsumeb. Karl Seifert's son Joachim has given permission for the naming of the mineral. The double name karlseifertite is proposed because the name seifertite (SiO2) corresponds to another mineral species.
The mineral and its name have been approved by the International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC), with proposal number IMA 2024-007 (Warr symbol: Karl). One holotype specimen and one cotype specimen are deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA, with catalogue numbers 76334 and 76335, respectively.
Karlseifertite was found on specimens from the Tsumeb mine, Tsumeb, Namibia (19°15′ S, 17°42′ E). Overviews of the mineralogy and geology of the Tsumeb deposit can be found in Gebhard (1999) and von Bezing (2007), amongst other publications. Karlseifertite occurs on fracture surfaces in germanite–chalcocite ore. The specimens come from the collection of Karl Seifert, who was mining engineer at Tsumeb from 1963 to 1967. The second oxidation zone of the deposit was being mined during that period, and that is almost certainly where specimens containing karlseifertite originated. Karlseifertite is a secondary oxidation-zone mineral.
Karlseifertite occurs in rosettes of thin hexagonal plates (bounded by the {001} and {100} forms) up to about 0.2 mm in diameter and usually less than 0.01 mm thick (Fig. 1). No twinning was observed. The colour is yellow, and the streak is pale yellow. The mineral has subadamantine lustre and is transparent. No fluorescence was observed in either long- or short-wave ultraviolet illumination. The Mohs hardness is probably about 4 by analogy with other members of the dussertite group. The tenacity is brittle, and the fracture is irregular. The density could not be measured because crystals exceed the density of available density fluids. The density calculated from the empirical formula and single-crystal cell is 4.993 g cm−3. At room temperature, karlseifertite is insoluble in dilute HCl.

Figure 1Rosette of karlseifertite plates on germanite on the holotype specimen (no. 76334). Field of view: 0.56 mm across.
Optically, karlseifertite crystals are uniaxial (+). Because of the thin platy habit, only ω could be measured. The ε−ω birefringence measured on a spindle stage using a Berek compensator is 0.004. The value of ω determined in white light is 1.890(5), and the calculated value of ε is 1.894. Pleochroism is slight with O (light yellow) and E (pale yellow): O > E. The Gladstone–Dale compatibility index (Mandarino, 1981) is −0.001 (superior) based on the empirical formula and the single-crystal cell.
Crystals of karlseifertite were analysed at Caltech on a JEOL JXA-iHP200F field-emission electron probe microanalyser (EPMA) in WDS (wavelength dispersive spectroscopy) mode (eight points). Analytical conditions were a 15 kV accelerating voltage, 5 nA beam current and 10 µm beam diameter. Insufficient material is available for the determination of H2O, so it is calculated based on the structure (O = 14 and As + S + W = 2). The crystals did not take a good polish, which, coupled with the thinness of the plates, resulted in the low analytical total. Analytical data are given in Table 1.
Table 1Analytical data (wt %) for karlseifertite.
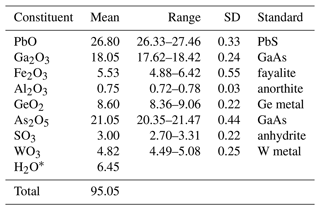
* Based on the structure (O = 14 and As + S + W = 2).
The empirical formula (based on 14 O apfu, atoms per formula unit), with cations allotted to structural sites, is Pb(GaGeFeAl)Σ2.97[(AsSW)Σ1.00O4]2(OH0.99)6. The simplified formula is Pb(Ga,Ge,Fe3+,Al)[(As,S,W)O4]2(OH)6, and the ideal formula is Pb(Ga2Ge)(AsO4)2(OH)6, which requires the following: PbO 27.93, Ga2O3 23.46, GeO2 13.09, As2O5 28.76, H2O 6.76, total 100 wt %.
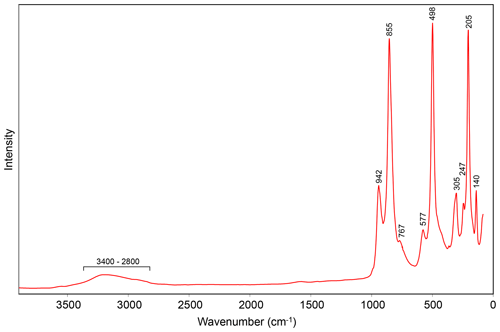
Raman spectroscopy was conducted on a Horiba XploRA PLUS using a 532 nm diode laser, 100 µm slit and 1800 grooves mm−1 diffraction grating, and a 100× (0.9 NA) objective lens. The spectrum from 3900 to 60 cm−1 is shown in Fig. 2.
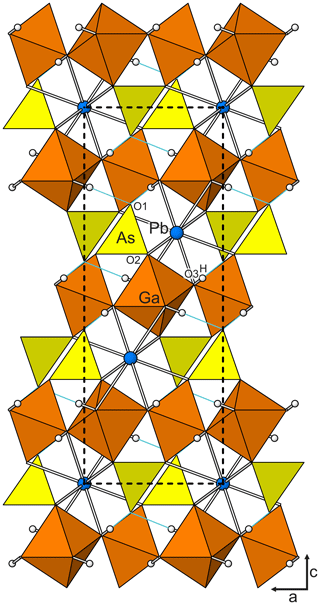
Figure 3The karlseifertite structure viewed down [010]. Hydrogen bonds are shown as light-blue lines. The unit-cell outline is shown with dashed lines.
The very broad band in the range ∼ 3400 to ∼ 2800 cm−1 is attributed to O–H stretching vibrations. The bands between ∼ 1000 and ∼ 700 cm−1 are likely due to the ν3 and ν1 stretching modes of AsO4 groups. The bands between ∼ 600 and ∼ 400 cm−1 are probably due to ν4 and ν2 bending modes of AsO4 groups. The bands at lower wavenumbers are attributable to various lattice vibrations.
6.1 X-ray powder diffraction
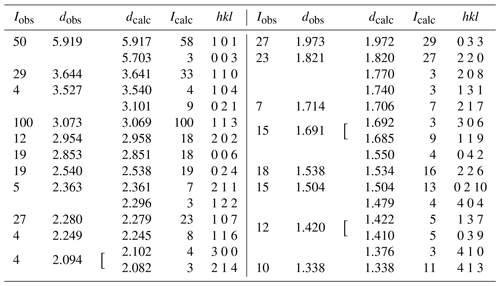
X-ray powder diffraction data were recorded using a Rigaku R-AXIS RAPID II curved-imaging-plate microdiffractometer with monochromatized MoKα radiation. A Gandolfi-like motion on the ϕ and ω axes was used to randomize the sample. Observed d values and intensities were derived by profile fitting using the JADE Pro software (Materials Data, Inc.). Data are given in Table 2. Refined trigonal unit-cell parameters (space group ) are a=7.280(5), c=17.121(12) Å and V=785.8(12) Å3.
6.2 Single-crystal diffraction
Single-crystal X-ray studies were also done using a Rigaku R-AXIS RAPID II curved-imaging-plate microdiffractometer with monochromatized MoKα radiation. The Rigaku CrystalClear software package was used for processing the structure data, including the application of an empirical absorption correction using the multi-scan method with ABSCOR (Higashi, 2001). The structure was solved using the intrinsic-phasing algorithm of SHELXT (Sheldrick, 2015a). SHELXL-2016 (Sheldrick, 2015b) was used for the refinement of the structure.
Karlseifertite has an alunite-supergroup structure (Bayliss et al., 2010) with the general formula . Based on the EPMA, the large 12-fold-coordinated D site in karlseifertite is occupied solely by Pb2+. The octahedrally coordinated G site is occupied by Ga3+, Ge4+, Fe3+ and Al3+. The tetrahedrally coordinated T site is occupied by As5+, S6+ and W6+. The X sites are occupied by O, with the X′ site corresponding to OH. With Pb, Ga and As assigned to the D, G and T cation sites, respectively, their occupancies refined to 0.99, 1.03 and 0.96, respectively. The refined site scattering for the G site (95.79 e) is significantly higher than that calculated from the EPMA (88.68 e), whereas the refined site scattering for the T site (159.59 e) is lower than that calculated from the EPMA (164.88 e). Although these differences could reflect real differences in the composition between the structure crystal and those analysed by the EPMA, we think it more likely that they are due to deficiencies in the structure data, considering the relatively high values for Rint and R1 and rather large electron density residuals of 6.98 e A−3 located 0.83 Å from Pb, 5.15 e A−3 located 0.83 Å from As, 3.76 e A−3 located 0.86 Å from Ga and 3.71 e A−3 located 0.78 Å from As. Note that the EPMA compositions for the sites were used for calculating the bond valences.
All sites were successfully refined anisotropically; however, the ellipsoid for the Pb site was markedly oblate (flattened in the c direction), indicative of shifting of the Pb away from the origin, as has been noted in the structures of most other Pb-bearing alunite-supergroup minerals. This is attributed to 6 s2 lone-pair electrons (see for example Mills et al., 2009). The largest electron density residual (6.98 e A−3), located 0.83 Å from the origin along z, may also be evidence of displacement of the Pb. Nevertheless, our attempts to refine the Pb at positions displaced from the origin were unsuccessful.
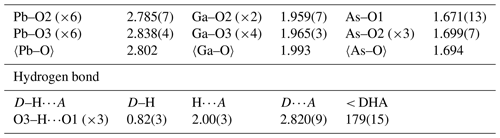
Difference Fourier synthesis revealed the likely H site associated with the O3 site. The H site was refined with a soft restraint of 0.82(3) Å on the O3–H distance and with the Ueq of the H set to 1.5 times that of O3. Data collection and refinement details are given in Table 3, atom coordinates and displacement parameters are given in Table 4, selected bond distances and angles are given in Table 5, and a bond-valence analysis is given in Table 6. The crystal structure is shown in Fig. 3.
Table 3Data collection and structure refinement for karlseifertite. SREF: single-crystal structure refinement. GoF: goodness of fit.

Notes: . GoF = S = . . ; , where a is 0.0911, b is 14.2 and P is .
Table 4Refined atom coordinates, displacement parameters (Å2) and site occupancies for karlseifertite.
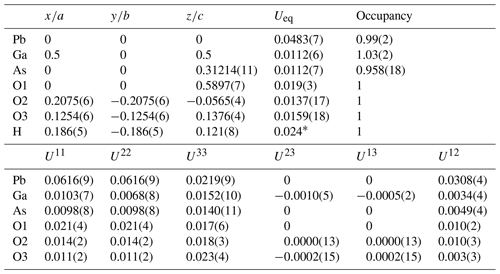
Table 6Bond-valence analysis for karlseifertite. Values are expressed in valence units (vu).
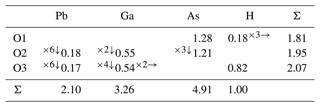
Bond-valence parameters are from Gagné and Hawthorne (2015). Hydrogen-bond valences are based on O–O bond lengths from Ferraris and Ivaldi (1988). The Ga and As sites were modelled using the EPMA compositions.
Karlseifertite is a new member of the dussertite group of the alunite supergroup. It is most closely related to galloplumbogummite, whose ideal formula is given as Pb(Ga,Al,Ge)3(PO4)2(OH)6 in the IMA master list; however, this is not a proper ideal formula. Schlüter et al. (2014) gave its formula as “Pb(Ga,Al)3−xGexH1−x(PO4)2(OH)6 for or Pb(Ga,Al)2Ge(PO4)2(OH)6 for the end member with x=1.” The latter formula is actually not an endmember formula but can be restated as PbGa2Ge(PO4)2(OH)6 for Ga > Al, which would correspond to the phosphate analogue of karlseifertite; however, x is 0.38 in the empirical formula of galloplumbogummite reported by Schlüter et al. (2014). Karlseifertite is also close to being the Ga analogue of segnitite, PbFe(AsO4)(AsO3OH)(OH)6, except for the essential presence of Ge4+ (rather than H+) in karlseifertite for charge balance. Note that Jambor et al. (1996) reported the occurrence of the unnamed Ga analogue of segnitite from Tsumeb with much lower Ge content than karlseifertite, presumably with the ideal formula PbGa3(AsO4)(AsO3OH)(OH)6.
Crystallographic data for karlseifertite are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-36-873-2024-supplement.
ARK oversaw the research, determined the physical and optical properties, did the Raman spectroscopy, did the X-ray diffraction studies and wrote the paper. JD conducted initial characterization studies on the mineral and identified it as a potentially new species. CM did the electron microprobe analyses.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “New minerals: EJM support”. It is not associated with a conference.
Reviewers Jochen Schlüter and Oleg Siidra are thanked for their constructive comments on the paper.
A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
This paper was edited by Sergey Krivovichev and reviewed by Jochen Schlüter and Oleg Siidra.
Bayliss, P., Kolitsch, U., Nickel, E. H., and Pring, A.: Alunite supergroup: recommended nomenclature, Mineral. Mag., 74, 919–927, 2010.
Ferraris, G. and Ivaldi, G.: Bond valence vs. bond length in O…O hydrogen bonds, Acta Crystallogr. B, 44, 341–344, 1988.
Gagné, O. C. and Hawthorne, F. C.: Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen, Acta Crystallogr. B, 71, 562–578, 2015.
Gebhard, G.: Tsumeb II. A Unique Mineral Locality, GG Publishing, Grossenseifen, Germany, ISBN 978-3925322037, 1999.
Hatert, F. and Burke, E. A. J.: The IMA–CNMNC dominant-constituent rule revisited and extended, Can. Mineral., 46, 717–728, 2008.
Higashi, T.: ABSCOR, Rigaku Corporation, Tokyo, 2001.
Jambor, J. L., Owens, D. R., Grice, J. D., and Feinglos, M. N.: Gallobeudantite, PbGa3[(AsO4),(SO4)]2(OH)6, a new mineral species from Tsumeb, Namibia, and associated new gallium analogues of the alunite-jarosite family, Can. Mineral., 34, 1305–1315, 1996.
Mandarino, J. A.: The Gladstone-Dale relationship: Part IV. The compatibility concept and its application, Can. Mineral., 19, 441–450, 1981.
Mills, S. J., Kampf, A. R., Raudsepp, M., and Christy, A. G.: The crystal structure of Ga-rich plumbogummite from Tsumeb, Namibia, Mineral. Mag., 73, 837–845, 2009.
Schlüter, J., Malcherek, T., and Mihailova, B.: Galloplumbogummite from Tsumeb, Namibia, a new member of the alunite group with tetravalent charge balance, Neues Jb. Miner. Abh., 2014, 301–309, 2014.
Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination, Acta Crystallogr. A, 71, 3–8, 2015a.
Sheldrick, G. M.: Crystal Structure refinement with SHELX, Acta Crystallogr. C, 71, 3–8, 2015b.
Von Bezing, L.: Namibia – Minerals and Localities, Bode Verlag GmbH, Haltern, ISBN 978-3925094897, 2007.
- Abstract
- Introduction
- Occurrence and associated minerals
- Physical and optical properties
- Chemical composition
- Raman and infrared spectroscopy
- Crystallography
- Discussion
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Occurrence and associated minerals
- Physical and optical properties
- Chemical composition
- Raman and infrared spectroscopy
- Crystallography
- Discussion
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Special issue statement
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement