the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Ellinaite, CaCr2O4, a new natural post-spinel oxide from Hatrurim Basin, Israel, and Juína kimberlite field, Brazil
Sergey N. Britvin
Felix V. Kaminsky
Richard Wirth
Elena N. Nigmatulina
Grigory A. Yakovlev
Konstantin A. Novoselov
Mikhail N. Murashko
Ellinaite, a natural analog of the post-spinel phase β-CaCr2O4, was discovered at the Hatrurim Basin, Hatrurim pyrometamorphic formation (the Mottled Zone), Israel, and in an inclusion within the super-deep diamond collected at the placer of the Sorriso River, Juína kimberlite field, Brazil. Ellinaite at the Hatrurim Basin is confined to a reduced rankinite–gehlenite paralava, where it occurs as subhedral grains up to 30 µm in association with gehlenite, rankinite and pyrrhotite or forms the rims overgrowing zoned chromite–magnesiochromite. The empirical formula of the Hatrurim sample is (Ca0.960FeNa0.012Mg0.003)0.992(Cr1.731VTiAl0.023TiO4. The mineral crystallizes in the orthorhombic system, space group Pnma, unit-cell parameters refined from X-ray single-crystal data: a 8.868(9), b 2.885(3), c 10.355(11) Å, V 264.9(5) Å3 and Z=4. The crystal structure of ellinaite from the Hatrurim Basin has been solved and refined to R1=0.0588 based on 388 independent observed reflections. Ellinaite in the Juína diamond occurs within the micron-sized polyphase inclusion in association with ferropericlase, magnesioferrite, orthorhombic MgCr2O4, unidentified iron carbide and graphite. Its empirical formula is Ca1.07(Cr1.71FeV0.06Ti0.03Al0.03Mg0.02Mn0.02)Σ1.93O4. The unit-cell parameters obtained from HRTEM data are as follows: space group Pnma, a 9.017, b 2.874 Å, c 10.170 Å, V 263.55 Å3, Z=4. Ellinaite belongs to a group of natural tunnel-structured oxides of the general formula AB2O4, the so-called post-spinel minerals: marokite CaMn2O4, xieite FeCr2O4, harmunite CaFe2O4, wernerkrauseite CaFeMn4+O6, chenmingite FeCr2O4, maohokite MgFe2O4 and tschaunerite Fe(FeTi)O4. The mineral from both occurrences seems to be crystallized under highly reduced conditions at high temperatures (>1000 ∘C), but under different pressure: near-surface (Hatrurim Basin) and lower mantle (Juína diamond).
- Article
(4239 KB) - Full-text XML
-
Supplement
(2389 KB) - BibTeX
- EndNote
The double oxide CaCr2O4 is known as an important component of composite materials explored in metallurgy and as ceramic materials (Róg et al., 2007). The α- and β-polymorphs of CaCr2O4, corresponding to its high- and low-temperature forms, demonstrate the promising magnetic and magnetoelectric properties (Hill et al., 1956; Pausch and Müller Buschbaum, 1974; Hörkner and Müller Buschbaum, 1976; Degterov and Pelton, 1996; Lee and Nassaralla, 1997; Damay et al., 2010; Toth et al., 2011; Zhai et al., 2016, and references herein). The β-polymorph belongs to a series of double oxides having the parent structure of CaFe2O4 (CF), which, altogether with CaTiO4 (CT) and CaMnO4 (CM), are gathered under the group name of post-spinel phases (Hill et al., 1956; Bright et al., 1958; Hörkner and Müller Buschbaum, 1976; Irifune et al., 1991; Kirby et al., 1996; Damay et al., 2010; Xue et al., 2021). These oxides are regarded as structural models for the high-pressure (HP) oxyspinels stable at the conditions of Earth's deep mantle (Chen et al., 2003a; Zhai et al., 2016, and references herein). Diverse synthetic compounds with post-spinel structure of the general formula AB2O4 are known, where A is Li, Na, Mg, Ca, Sr, Ba, La and Eu and B is Ti, V, Cr, Mn, Fe, Ru, Rh, Al, Ga, In, Tl, Sc, Y and REE (Shizuya et al., 2007).
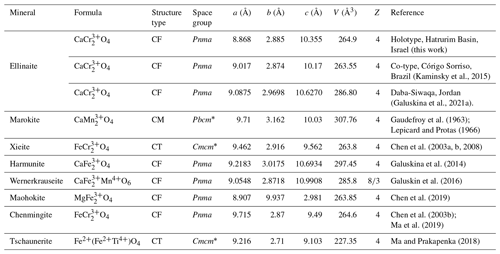
In nature, seven post-spinel minerals were described prior to 2019: marokite CaMnO4 (Gaudefroy et al., 1963; Lepicard and Protas, 1966), xieite FeCrO4 (Chen et al., 2003a, b, 2008), harmunite CaFeO4 (Galuskina et al., 2014), wernerkrauseite CaFeMn4+O6 (Galuskin et al., 2016), chenmingite FeCrO4 (Chen et al., 2003b; Ma et al., 2019), maohokite MgFeO4 (Chen et al., 2019) and tschaunerite Fe2+(Fe2+Ti4+)O4 (Ma and Prakapenka, 2018). Xieite, chenmingite, maohokite and tschaunerite are considered to be HP polymorphs, whereas other minerals may be formed under low-pressure conditions. Xieite, a HP polymorph of chromite FeCr2O4, was found in the shock veins of the Suizhou L6 meteorite, China (Chen et al., 2008), and maohokite, a HP polymorph of magnesioferrite, was reported in shocked gneiss from the Xiuyan crater in China (Chen et al., 2019). Chenmingite and tschaunerite were observed as shock-induced phases in the Tissint and Shergotty martian meteorites (Ma et al., 2019; Ma and Prakapenka, 2018). Harmunite CaFe2O4 and wernerkrauseite CaFeMn4+O6 are more common of Ca-rich high-temperature low-pressure pyrometamorphic rocks (Chesnokov et al., 1991; Nigmatulina, 2006; Nigmatulina and Nigmatulina, 2009; Galuskina et al., 2014; Sharygin, 2015; Galuskin et al., 2016). The Ca–Cr oxide phase, chemically and structurally similar to synthetic β-CaCr2O4, and post-spinel (Mg,Mn)(Cr,Fe)2O4 were found as potentially new mineral species in a microinclusion in a diamond from Brazil (Kaminsky et al., 2015). Unfortunately, the HRTEM data obtained and very small sizes were insufficient to approve these phases as new minerals.
This work covers all data obtained for a new mineral ellinaite, a natural analog of β-CaCr2O4, coming from two diverse localities: rankinite–gehlenite paralava at Hatrurim Basin, Hatrurim pyrometamorphic formation (Mottled Zone), Israel (holotype, Sharygin, 2019; Sharygin et al., 2019a), and a micron-sized inclusion in a diamond from the Sorriso River, Brazil (co-type; Kaminsky et al., 2015). The mineral was approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) as a new mineral species in December 2019, IMA 2019-091 (Sharygin et al., 2020). After the IMA registration, the same mineral has also been identified in varicolored spurrite marbles at Tulul Al Hammam, Daba-Siwaqa, Hatrurim pyrometamorphic formation, central Jordan (Galuskina et al., 2021a).
Ellinaite is named in honor of Ellina Vladimirovna Sokol (b. 1961) from IGM, Novosibirsk, Russia. Ellina (Ella) Sokol is a well-known Russian mineralogist and petrologist, who specialized in the studies of pyrometamorphic and combustion metamorphic rocks around the world, including the Hatrurim Formation (Mottled Zone) rocks, Israel–Jordan (Sokol et al., 2002, 2005, 2008, 2010, 2011, 2012, 2014a, b, 2015, 2017, 2019a, b, 2020; Khoury et al., 2016; Seryotkin et al., 2019; Sharygin et al., 2006, 2008, 2019b; Vapnik et al., 2007; Zateeva et al., 2007, and many other works).
An individual grain of ellinaite from rankinite–gehlenite paralava, Hatrurim Basin, Israel (sample MP-2013-6, used for many studies), was deposited in the collections of the A.E. Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia, with the registration number 5439/1 (holotype). Another grain of ellinaite from this Hatrurim paralava (in polished thin section) is in the collections of the Central Siberian Geological Museum at V.S. Sobolev Institute of Geology and Mineralogy (IGM), Siberian Branch of the RAS, Novosibirsk, Russia (catalogue number VII-102/1, holotype). The co-type sample of ellinaite from Córigo Sorriso, Mato Grosso State, Brazil (TEM foil from diamond), is located in the scientific collection of F.V. Kaminsky (V.I. Vernadsky Institute of Geochemistry and Analytical Chemistry, Moscow, Russia).
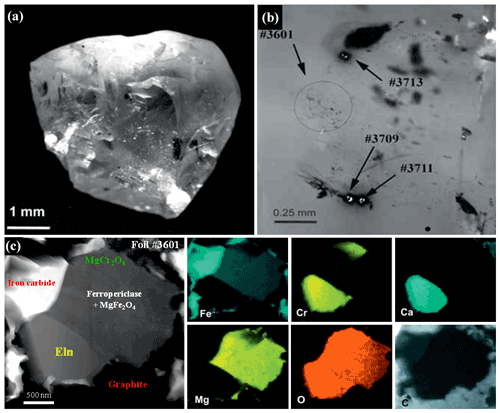
Figure 1(a) General view of diamond #8-108, Brazil. (b) Positions of TEM foils in the diamond. (c) Polyphase mineral inclusion with ellinaite, foil #3601, TEM image and elemental maps. Symbols: Eln – ellinaite; MgCr2O4 – orthorhombic MgCr2O4; MgFe2O4 – magnesioferrite; Iron carbide – Fe2(C,N), Fe3(C,N) or Fe7(C,N)3. Data were adapted from Kaminsky et al. (2015).
Natural ellinaite was first found in a polyphase inclusion within diamond #8-108 (foil #3601) (Kaminsky et al., 2015), collected from gravels of the Córigo Sorriso (Sorriso River, a right tributary of Rio Aripuanã), Juína kimberlite field, Mato Grosso State, Brazil (11∘20′ S, 59∘11′ W). It should be noted that the Juína kimberlite field is a well-known occurrence of the super-deep diamonds from both alluvial and primary kimberlite sources (Harte et al., 1999; Hutchison et al., 2001; Hayman et al., 2005; Kaminsky et al., 2001, 2009, 2013, 2015; Walter et al., 2008; Bulanova et al., 2010, and references herein).
Unfortunately, no new data for ellinaite in the Brazilian diamond have been obtained since the IMA registration of this mineral (Sharygin et al., 2020). As a result, the text below and figures provided here for the Brazilian ellinaite represent a compilation of data adapted from Kaminsky et al. (2015).
Within the polyphase inclusion ellinaite is associated with ferropericlase (+magnesioferrite), orthorhombic MgCr2O4, iron carbide and graphite (Figs. 1 and S1 in the Supplement). It occurs as a 2 × 1 µm subhedral grain. The EDX TEM spectrum for ellinaite (Fig. S1) was obtained by applying the TIA™ software package and using the kAB factors from the software. The quantitative results were normalized to the total sum of 100 %. The approximate uncertainties are ±3 % for concentrations in the range 30 at %–50 at %, ±6 %–12 % for concentrations between 5 at %–25 at %, ±12 %–25 % for concentrations between 1 at %–5 at % and ±25 %–100 % for concentrations below 1 at %. The composition (in at %, without oxygen) is Ca = 35.72, Cr = 57.02, Fe = 1.93, Mg = 0.79, Mn = 0.69, Al = 0.80, Ti = 1.09 and V = 1.96. The empirical formula calculated on the basis of three cations and four oxygens is Ca1.07(Cr1.71FeV0.06Ti0.03Al0.03Mg0.02Mn0.02)Σ1.93O4.
The unit-cell parameters were calculated from HRTEM data (see Table 5 in Kaminsky et al., 2015) using high-resolution images and fast Fourier transform (FFT) software packages (Fig. S1) and gave the following results: orthorhombic symmetry, space group Pnma (#62), a=9.017 Å, b=2.874 Å, c=10.170 Å, V=263.55 Å3 and Z=4.
Double-polished sections (∼50–100 µm in thickness) of the Hatrurim rankinite–gehlenite paralava (sample MP-2013-6, Fig. 2) were used for optical examination in transmitted and reflected light and for other studies.
Identification of ellinaite and related minerals in the Hatrurim rankinite–gehlenite paralavas was based on energy-dispersive spectra (EDS), back-scattered electron (BSE) images and elemental mapping (EDS system), using a TESCAN MIRA 3MLU scanning electron microscope equipped with an INCA Energy 450 XMax 80 microanalysis system (Oxford Instruments Ltd., Abingdon, UK) at the IGM, Novosibirsk, Russia. EDS analyses of minerals were operated at an accelerating voltage of 20 kV and a probe current of 1 nA in high-vacuum mode and at an accumulation time of 20–40 s. Synthetic compounds, pure metals and minerals were used as reference standards for the elements SiO2 (Si and O), Al2O3 (Al), diopside (Mg and Ca), albite (Na), orthoclase (K), Ca2P2O7 (P), BaF2 (Ba and F), Cr2O3 (Cr), CsRe2Cl6 (Cl), LaPO4 (La), CePO4 (Ce), SrF2 (Sr), metallic Nb, Ti, Ta, Zr, Fe, Mn, Zn and V. Matrix effects were corrected using the XPP algorithm, implemented in the software of the microanalysis system. Metallic Co served for quantitative optimization (normalization to probe current and energy calibration of the spectrometer).
Electron microprobe analyses (EMPA-WDS) of ellinaite and related minerals were done at the IGM, using a JEOL JXA-8100 electron microprobe (Jeol Ltd., Tokyo, Japan). Grains (sizes >5 µm) previously analyzed by EDS were selected for this purpose. Operating conditions were as follows: beam diameter of 1–2 µm, accelerating voltage of 20 kV and beam current of 15 nA, and counting time of 15 s (10 s – counting of backgrounds; 5 s – counting of peak for element). Pyrope (Si, Al, Mg, Fe), ilmenite (Ti), chromite (Cr), Sr-silicate glass (Sr), zircon (Zr), baryte (S), wollastonite (Ca), spessartine (Mn), albite (Na), chlorapatite (P, Cl), NiFe2O4 (Ni) and V2O5 (V) were used as standards during measuring. Precision of analysis for major elements was better than 2 % relative. Detection limits for elements were as follows (in parts per million, ppm): Si – 401, Ti – 182, Al – 303, Fe – 156, Mg – 309, Ca – 116, Cr – 192, Mn – 133, Sr – 766, Na – 239, K – 101, Cl – 98, Ni – 162 and S – 234. Data reduction was performed using a PAP routine (Pouchou and Pichoir, 1985). Overlap corrections were done for the following elements: SiKα–SrLα, TiKβ–VLα and CrKβ–MnKα.
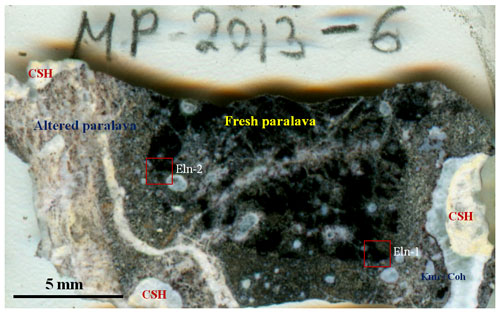
Figure 2General view of gehlenite-rankinite paralava fragment, containing ellinaite (thin section, sample MP-2013-6). CSH – hydrated calcium silicates; Kmc+Coh – kamacite–cohenite association. Red squares – areas of individual grains of ellinaite (Eln-1 and Eln-2).
Reflectance data for ellinaite were obtained using a microscope spectrophotometer LOMO MSP-R equipped with spectrophotometric attachment PEI “R928” (Hamamatsu, Japan) at the South Urals Federal Research Center of Mineralogy and Geoecology, Miass, Russia. Measurements were done in the air using a ×40 objective with numerical aperture of 0.65: photometric diaphragm – 0.3 mm, size of analyzing area – 0.007 mm, diffraction grating – 600 grooves per millimeter, spectral interval – 6 nm, voltage for PEI – 450 V and standard – elemental silicon. Measurements were provided for the 400–700 nm range.
Single-crystal data were collected by means of a Bruker Kappa APEX DUO CCD diffractometer using MoKα radiation. The crystal structure of ellinaite has been solved by the dual space method and refined to R1=0.059 using the SHELX-2014 set of programs (Scheldrick, 2015) via the Olex2 v.1.2.8 graphical user interface (Dolomanov et al., 2009). Further details of data collection and structure refinement can be retrieved from the CIF attached to the Supplement.
The Raman spectra for ellinaite were recorded on a LabRAM HR 800 mm (HORIBA Scientific Ltd.) spectrometer equipped with a 1024 pixel LN/CCD detector and coupled to an Olympus BX40 confocal microscope (×100 objective) at the IGM. A semiconductor laser emitting at 514.5 nm with a nominal output power of 50 mW was used for excitation. In each case, 20 spectra were recorded for 20 s each at a hole diameter of 200 µm and a resolution of 0.5 cm−1 and then integrated. Most spectra were recorded between 100 and 1400 cm−1. The monochromator was calibrated using the 520.7 cm−1 Raman line of elemental Si.
Electron backscatter diffraction (EBSD) studies were provided for one grain of ellinaite. The sample containing ellinaite and intended for EBSD studies was subjected to polishing by BuehlerMasterMet2 non-crystallizing colloidal silica suspension (0.02 µm). EBSD measurements were carried out by means of a FE-SEM ZEISS SIGMA VP scanning electron microscope equipped with an HKL Technology Nordlys HKL EBSD, operated at 20 kV and 1.4 nA in focused beam mode with a 70∘ tilted stage at the NANOTECH Center, Ural Federal University, Ekaterinburg, Russia. Structural identification of ellinaite was performed by matching its EBSD patterns with the reference structural models using the program FLAMENCO. The structural data for synthetic β-CaCr2O4 (Damay et al., 2010) and α-CaCr2O4 (Pausch and Müller Buschbaum, 1974) were used for the Kikuchi pattern simulation and comparison.
Ellinaite was observed in one of the rankinite–gehlenite paralavas, which were first found in 2011 in the southern part of the Hatrurim Basin, the largest combustion metamorphism complex of the Hatrurim Formation (also known as the “Mottled Zone”) within Israel (Gross, 1977; Burg et al., 1992; Vapnik et al., 2007). The paralava with ellinaite (sample MP-2013-6) was found as a boulder in the dry Zohav wadi (nahal Zohav), near the Arad – Dead Sea road (31∘09′47′′ N, 35∘17′57′′ E). Similar paralavas with abundant phosphides are also known in the midstream of the Halamish wadi (nahal Halamish, neighboring nahal Zohav) in the Hatrurim Basin (Britvin et al., 2015, 2017, 2019a–d, 2020a–c, 2021a. b).
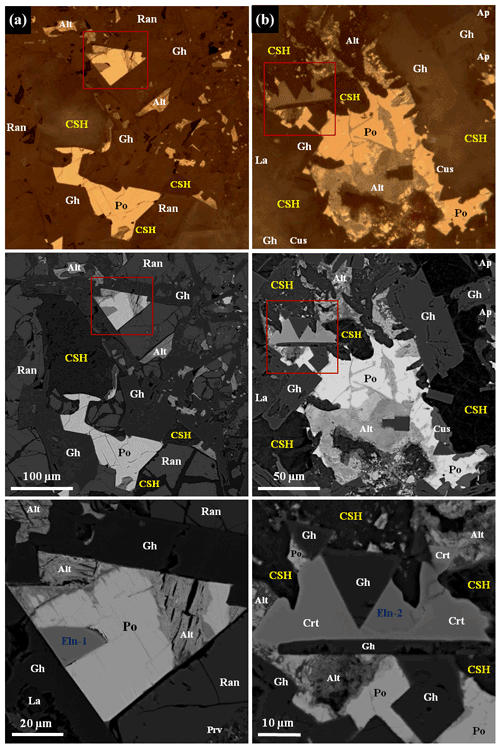
Figure 3Areas with ellinaite in gehlenite-rankinite paralava (a–b), Hatrurim Basin, Israel. BSE and reflected light images. Symbols: Eln-1, Eln-2 – ellinaite; Po – Cr-rich pyrrhotite; Gh – gehlenite; Ran – rankinite; La – larnite–flamite; Prv – Si-rich perovskite; Alt – alteration products after pyrrhotite; Ap – fluorapatite; Cus – cuspidine; Crt – chromite–magnesiochromite; CSH – hydrated calcium silicates. See Figs. 2, 4, S2 and S3 for details.
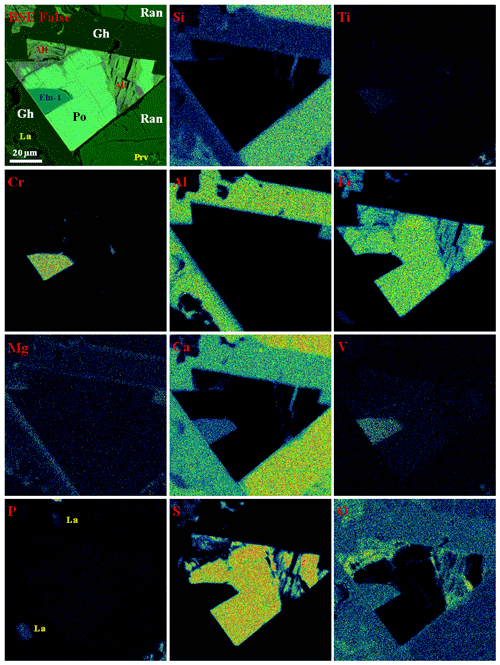
Figure 4Elemental maps of mineral association with ellinaite-1 grain from gehlenite-rankinite paralava, Hatrurim Basin, Israel. Symbols: Eln-1 – ellinaite; Po – Cr-rich pyrrhotite; Gh – gehlenite; Ran – rankinite; La – larnite–flamite; Prv – Si-rich perovskite; Alt – alteration products after pyrrhotite. See Figs. 3a and S2 for details.
The rock is moderately to highly weathered and brecciated and has the appearance of a “pseudoconglomerate”, in which individual fragments are “cemented” and cross-cut by a carbonate–gypsum–Ca–hydrosilicate matrix. In general the individual fragments are altered in different degrees and contain abundant vesicles now partially filled with Ca hydrosilicates. The alteration degree is indicated by color change (from dark to white) from the center to rim (Fig. 2). The dark parts of some fragments are less weathered and consist of gehlenite, rankinite, larnite, flamite, cuspidine, Cr-rich pyrrhotite, fluorapatite and Si–Cr–V-rich perovskite as essential minerals. Minor and accessory phases are represented by chromite–magnesiochromite, wüstite–magnesiowüstite, wollastonite, Ti-rich cuspidine, ellinaite, Ba-rich djerfisherite minerals, magnetite, unidentified Ca–Fe silicate Ca4Fe2(SiO4)3 and oxysulfide close to CaFe3S2O5, the (Ni,Fe)2As phase, pentlandite, native iron, cohenite, schreibersite Fe3P, and barringerite Fe2P (e.g., Britvin et al., 2015, 2017). Crystallization of the paralava likely occurred under highly reduced conditions that are determined by the chemistry of the essential minerals: Ca silicates and perovskite are very poor in Fe, whereas sulfides (pyrrhotite, Ba-rich phases), chromite, wüstite, native iron and phosphides are the main carriers of Fe. A list of mineral phases found in the rankinite–gehlenite paralava with ellinaite (sample MP-2013-6) and chemical composition of essential minerals are given in Tables S1 and S2. The metal-phosphide mineralization is mainly localized along the boundary of rock fragments with the carbonate–gypsum–Ca–hydrosilicate matrix, rarely around vesicles in the paralava. It forms rounded isolations varying in size from 10 µm to 1–2 mm and modal composition. Namely, in such associations the new phosphide minerals (murashkoite FeP, transjordanite Ni2P, zuktamrurite FeP2, negevite NiP2, halamishite Ni5P4, polekhovskyite MoNiP2, nazarovite Ni12P5) were first found in the Halamish wadi, Hatrurim Basin (Britvin et al., 2015, 2017, 2019a–d, 2020a–c). The following metal-phosphide assemblages with Cr–V-rich pyrrhotite are most common in the Zohav paralava: kamacite, steadite (Fe–P eutectics, mainly kamacite + schreibersite Fe3P) + kamacite, barringerite Fe2P + steadite, schreibersite + barringerite, barringerite + murashkoite and murashkoite + transjordanite. Other phosphide phases, cohenite, graphite, copper, Mo-rich phases, unidentified phosphates and daubreelite sometimes occur in such assemblages. The formation of paralava and metal-phosphide mineralization appears to be unrelated in that the metal-phosphide assemblage was probably formed in the solidified paralava from reduced gases (Britvin et al., 2015, 2017, 2019a–d). In addition to the metal phosphide and sulfide association, the new Fe–Ni-rich phosphates were found in the carbonate–gypsum–Ca–hydrosilicate matrix as alteration products of phosphides (phosphocyclite-(Fe) Fe(P4O12) (IMA 2020-087), phosphocyclite-(Ni) Ni(P4O12) (IMA 2020-088), beershevaite CaFe(PO4)3O (IMA 2020-095a), lisanite CaNi2+P2O7 (IMA 2021-014), shasuite CaNi(P2O7)2 (IMA 2021-20), nabateaite Fe(P2O7) (IMA 2021-26) and samraite Ni(P2O7) (IMA 2021-29)) in the Halamish rocks (Britvin et al., 2021a).
Ellinaite is very rare mineral in the Hatrurim Basin, and only two grains were observed in a dark unaltered part of the rankinite–gehlenite paralava (Fig. 2). It forms subhedral opaque grains in association with gehlenite, rankinite and pyrrhotite (size – 30 × 20 µm, ellinaite-1 grain, Figs. 3a, 4 and S2) or is intergrown with chromite–magnesiochromite (size – 10 × 10 µm, ellinaite-2 grain, Figs. 3b and S3). In reflected light and in BSE images this phase resembles perovskite and chromite, which are more abundant in the studied paralava. Mineral relations indicate that ellinaite crystallized after gehlenite, rankinite and chromite, but before pyrrhotite (Fig. 3).
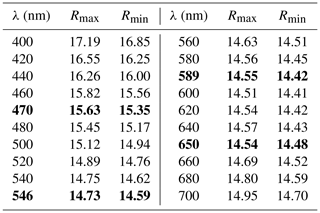
The color of ellinaite is black, and the color of the powdered mineral is black. It is brittle and has submetallic luster. Its hardness is ≈4.5–5 (Mohs), which is close to neighboring chromite (Fig. 3b). No cleavage and parting were observed; fracture is uneven. Microhardness and density were not measured directly because of the small grain size of ellinaite. Density calculated from unit-cell dimensions and results of electron-microprobe analyses is 5.217 g cm−3. Ellinaite is weakly distinguishable optically from the black chromite–magnesiochromite in reflected light and in BSE images (Figs. 3b and S3). Under reflected light it is gray with a blue tint and shows weak red-brown internal reflections. Bireflectance and pleochroism are not observed; anisotropy is weak. The reflectance data for the mineral in air are given in Table 1. Reflectance percentages for the four Rmax and Rmin COM (Commission on Ore Mineralogy) wavelengths are 15.63, 15.35 (470 nm); 14.73, 14.59 (546 nm); 14.55, 14.42 (589 nm); and 14.54, 14.48 (650 nm).
The empirical formula of ellinaite calculated on the basis of three cations and four oxygens and WDS-EDS data (n=48) is (Ca0.960FeNa0.012Mg0.003)0.992(Cr1.731VTiAl0.023Ti)2.008O4. The average data for two ellinaite grains are given in Table 2, and the database for the Hatrurim Basin ellinaite is represented in Tables S3 and S4 (Supplement). Taking into account the reduced conditions for paralava crystallization (presence of native iron, cohenite and Cr-rich pyrrhotite) and excess of positive charge in a calculated formula, a dominant three-valence state is suggested for Ti. Thus, the simplified formula for the Hatrurim ellinaite may be expressed as Ca(Cr3+,V3+,Ti3+)2O4 and the ideal formula as CaCr2O4, which requires (in wt %) CaO 26.95, Cr2O3 73.05 and total 100.00. Alternatively, a formula version with minor vacancy at the Ca site and tetravalent Ti may also be considered. In this case the empirical formula (on the basis of four oxygens) is (Ca0.952FeNa0.012Mg0.003)0.983(Cr1.716VTiAl0.023)1.991O4.
Table 2Chemical composition (in wt %) for ellinaite from rankinite–gehlenite paralava (sample MP-2013-6), Hatrurim Basin, in comparison with ellinaite from the Daba-Siwaqa spurrite marble and the Brazilian diamond and ideal composition CaCr2O4.
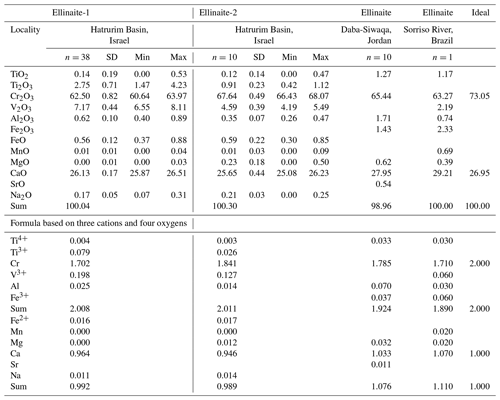
For Hatrurim Basin, SiO2, NiO and SrO are below detection limits (<0.005 wt %). Formula based on three cations and four oxygens. Ti2O3 and TiO2 is calculated by charge balance. Ellinaite-1 – WDS + EDS data; Ellinaite-2 – EDS data. The database for the Hatrurim Basin ellinaite is given in Tables S3 and S4. Composition of ellinaite in the Brazilian diamond (Kaminsky et al., 2015) are recalculated in wt % of oxides. Data for ellinaite at Tulul Al Hammam, Daba-Siwaqa, Jordan, are quoted from Galuskina et al. (2021a).
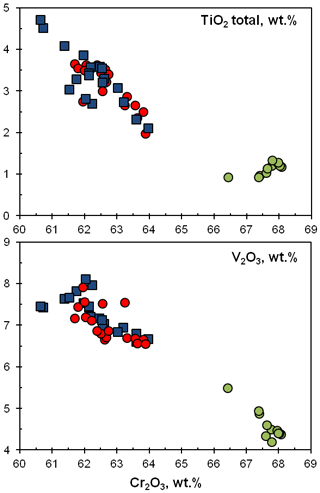
Figure 5Chemical variations (in wt %) of TiO2 and V2O3 versus Cr2O3 in ellinaite from the Hatrurim Basin. Ellinaite-1 grain: blue squares – WDS data; red circles – EDS data. Green circles – EDS data for the ellinaite-2 grain. For the database for the Hatrurim Basin ellinaite, see Tables S3 and S4.
The main variation (4 wt %–7 wt %) of the Hatrurim ellinaite composition is in the amounts of TiO2, V2O3 and Cr2O3 (Fig. 5). Both ellinaite grains are drastically different in composition (Table 1): (Ca0.964FeNa0.011)0.992(Cr1.702VTiAl0.025Ti)2.008O4 (ellinaite-1) and (Ca0.946FeNa0.014Mg0.012)0.989(Cr1.841VTiAl0.014Ti)2.011O4 (ellinaite-2). The variations within individual grains are not very significant (Fig. 5) and may indicate a slight chemical heterogeneity like that of core-to-rim variation. It should be noted that such inhomogeneous distribution is weakly highlighted in elemental maps (Figs. 4, S2 and S3). For example, the Ti-richest (TiO2 total – 4.7 wt %) and V-richest (V2O3 – 8.1 wt %) compositions within the ellinaite-1 grain are (Ca0.960FeNa0.009Mn0.001Mg0.001)0.991(Cr1.653VTiAl0.028)2.009O4 and (Ca0.975FeNa0.006)0.994(Cr1.686VTiAl0.023)2.006O4 (Table S3). Negative correlations of Cr with Ti and V (Fig. 5) strongly suggest the occurrence of limited isomorphous substitutions according to possible schemes: Cr (V3+,Ti3+) and 2Cr V Ti3+. Unfortunately, we have no possibility to check the valence state of Ti and other elements due to the small amount of ellinaite present.
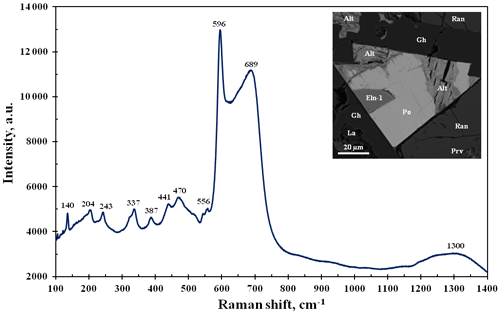
Figure 6BSE image and Raman spectrum for ellinaite from the Hatrurim Basin, Israel (see also Fig. 3). Symbols: Eln-1 – ellinaite-1 grain; Po – Cr-rich pyrrhotite; Gh – gehlenite; Ran – rankinite; La – larnite; Prv – Si-rich perovskite; Alt – alteration products after pyrrhotite.
Ellinaite from the Hatrurim Basin paralava essentially differs in composition from the same mineral in the Brazilian diamond, Ca1.07(Cr1.71FeV0.06TiAl0.03Mg0.02Mn0.02)Σ1.93O4 (Kaminsky et al., 2015), and in the Jordanian spurrite marble, (Ca1.033Mg0.032Sr0.011)1.076(Cr1.785FeTiAl0.070)Σ1.924O4 (Galuskina et al., 2021a). The Hatrurim ellinaite has higher concentrations of Ti (dominantly Ti3+) and V and a lower amount of Fe (dominantly Fe2+) compared with the other two occurrences. The Brazilian and Jordanian ellinaites are characterized by a high concentration of Fe3+ and low content of total Ti4+. In the Brazilian sample the valence state of Fe (as Fe3+) in ellinaite is anomalous considering the presence of associated Fe carbide and ferropericlase in the diamond-hosted inclusion (Kaminsky et al., 2015). In the Jordanian case we do not see any contradictions since Fe-oxide minerals (brownmillerite, srebrodolskite, hematite) and garnet-supergroup minerals (mainly andradite and priscillagrewite-(Y)) in spurrite marble are dominated by Fe3+ (Galuskina et al., 2021a, b).
7.1 Raman spectroscopy
The Raman spectra for the Hatrurim ellinaite show the complete band set common to synthetic β-CaCr2O4 (Zhai et al., 2016). However, in addition to the strong 596 cm−1 band, the Hatrurim phase indicates the strong and broad band at 690 cm−1 with respect to the synthetic compound (Fig. 6). This seems to be related to crystallographic orientation of the natural sample. The appearance and disappearance of some bands and their shifting and intensity change in the dependency on orientation have been shown for the Raman spectra of synthetic CaFe2O4 (Kolev et al., 2003). The vibration modes of the Raman spectrum of ellinaite may be interpreted by analogy with the Raman spectra of harmunite and wernerkrauseite and related synthetic compounds (Kolev et al., 2003; Galuskina et al., 2014; Galuskin et al., 2016; Zhai et al., 2016). Observed Raman bands are (cm−1) ∼1300 (combination first-order phonons Ag in the 680–700 range); 689, 598 and 556 (Ag – stretching modes); 470 and 441 (B2g and Ag – tilting and bending modes); 387, 337 and 324 (Ag, B1g and B3g – rotating modes); 243 and 204 (Ag); 140 (Ag and B2g); and 123 and 107 (Ag) (Ca-related vibrations). In general, Raman spectra of ellinaite from Hatrurim Basin are similar to those of ellinaite from Jordan (Galuskina et al., 2021a).
7.2 EBSD study
Before single-crystal X-ray study, we attempted to obtain the EBSD data for ellinaite. Two structural patterns of synthetic CaCr2O4 were used for EBSD comparison studies: α-CaCr2O4 (Toth et al., 2011) and β-CaCr2O4 (Damay et al., 2010). We determined a good diffraction pattern, but, unfortunately, it was not indexed in both comparison structural models. This appears to have been due to unsuccessful orientation of the ellinaite crystal, although the data obtained indicated an orthorhombic symmetry. The forced Kikuchi pattern using the β-CaCr2O4 model (Damay et al., 2010) outlined strong mean angular deviation for some diffraction lines (Fig. S4).
7.3 X-ray data for ellinaite
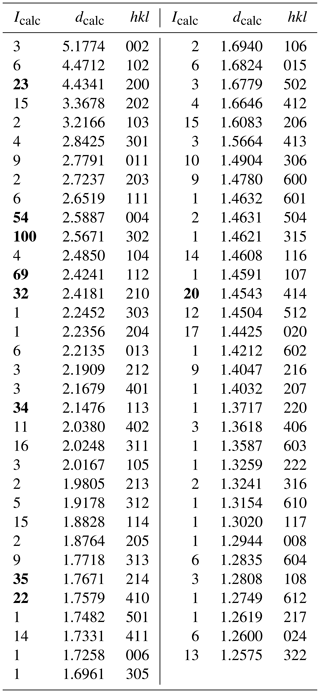
Single-crystal X-ray studies for the Hatrurim ellinaite indicate that ellinaite is related to the orthorhombic crystal system: space group Pnma (#62), a=8.868(9) Å, b=2.885(3) Å, c=10.355(11) Å, V=264.9(5) Å3 and Z=4. X-ray powder diffraction data could not be obtained because of scarcity of material. Powder diffraction pattern calculated on the basis of single-crystal structural data and chemical composition is given in Table 3. Data were calculated using the STOE WinXPOW v.2.02 software (Stoe & Cie, 2006).
7.4 Crystal structure of ellinaite
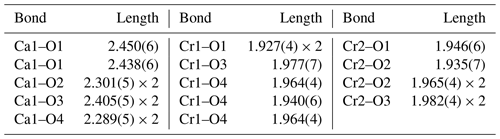
The crystal structure of ellinaite has been solved by the dual space method and refined to R1=0.059 using the SHELX-2014 set of programs (Scheldrick, 2015) via Olex2 v.1.2.8 graphical user interface (Dolomanov et al., 2009). The data collection and structure refinement details are summarized in Table 4. Fractional atomic coordinates, displacement parameters and selected bond lengths for the crystal structure of ellinaite are given in Tables 5 and 6.
Table 4Crystal parameters, data collection and structure refinement details for the holotype ellinaite (Hatrurim Basin, Israel).
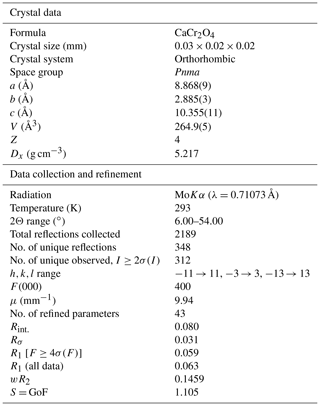
The ellinaite-1 crystal was used.
Table 5Fractional atomic coordinates and displacement parameters (Å2) for ellinaite (Hatrurim Basin).
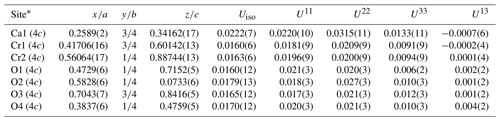
* Site multiplicities and Wyckoff symbols are given in parentheses. U12 and U23 are equal to zero by default.
In general, the crystal structure of ellinaite may be represented as a framework built up of two types of the distorted [Cr3+O6] octahedra (Cr1 and Cr2), sharing common edges and corners and containing the system of channels (tunnels) propagated along the b axis. The channels are occupied by calcium ions (Fig. 7). The framework is composed of two types of the distorted [Cr3+O6] octahedra (Cr1 and Cr2), which form double chains, connected by common oxygen corners. The distorted octahedra [Cr3+O6] in the chains have different average distances: Cr1–O = 1.950(3) Å and Cr2–O = 1.962(9) Å (Table 6). Owing to the specific connection of the chains, each tunnel has a cross section of a distorted trigonal prism (Fig. 7).
Ellinaite is related to multiple oxides with the general formula AB2O4. However, ellinaite does not belong to the spinel subgroup of the spinel supergroup (Biagioni and Pasero, 2014; Bosi et al., 2019) because it has a tunnel structure (post-spinel structure). Currently, minerals with this structure are regarded as belonging to the hypothetical marokite subgroup, which now includes eight minerals: marokite CaMnO4 (Gaudefroy et al., 1963; Lepicard and Protas, 1966), xieite FeCrO4 (Chen et al., 2003a, b, 2008), harmunite CaFeO4 (Galuskina et al., 2014), wernerkrauseite CaFeMn4+O6 (Galuskin et al., 2016), chenmingite FeCrO4 (Chen et al., 2003b; Ma et al., 2018), maohokite MgFeO4 (Chen et al., 2019), tschaunerite Fe2+(Fe2+Ti4+)O4 (Ma and Prakapenka, 2018) and ellinaite CaCr2O4 (this work). Comparative crystallographic data for these minerals are given in Table 7. Moreover, the orthorhombic MgCr2O4 phase (a=9.467 Å, b=2.905 Å, c=9.550 Å), which is associated with ellinaite in the Brazilian diamond-hosted inclusion (Kaminsky et al., 2015), appears to be related to the marokite supergroup. This phase may be considered a Mg analog of chenmingite FeCr2O4 observed as a shock-induced phase in the Tissint martian meteorite (Chen et al., 2003b; Ma et al., 2019) or xieite FeCr2O4 found in the shock veins of the Suizhou L6 meteorite (Chen et al., 2003a, b, 2008).
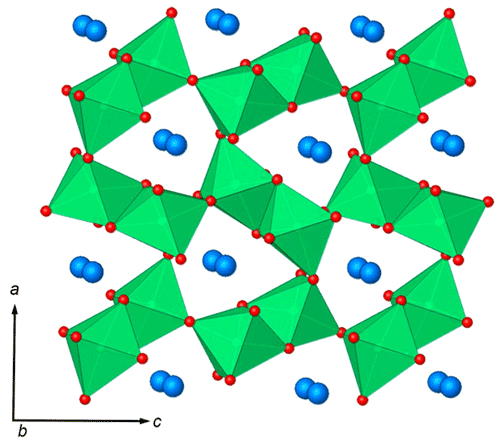
Figure 7The crystal structure of ellinaite. A framework composed of edge- and corner-sharing [CrO6] octahedra (green) contains the system of channels propagated along the b axis. The channels are occupied by calcium ions (blue spheres).
Table 7Comparative crystallographic data for ellinaite and related post-spinel minerals.
Tunnel structures: CF – CaFeO4; CT – CaTiO4; CM – CaMnO4. * Transformed to standard setting.
Initially high content of Cr in the sedimentary protolith (100–480 ppm Cr) was favorable to high abundance of Cr in the Hatrurim Basin pyrometamorphic rocks (100–800 ppm Cr) and then the appearance of Cr-rich minerals in them (Geller et al., 2012; Seryotkin et al., 2019; Sokol et al., 2019a). In the rankinite–gehlenite paralava this is fixed in the presence of Cr-dominant oxides (chromite – 62.2 wt %–64.3 wt % Cr2O3, magnesiochromite – 61.3 wt %–62.3 wt % Cr2O3, ellinaite – 60.6 wt %–68.6 wt % Cr2O3) and Cr-containing oxides (Si-rich perovskite – 1.6 wt %–2.8 wt % Cr2O3, magnetite – up to 3.0 wt % Cr2O3) and sulfides (pyrrhotite – 0.0 wt %–2.4 wt % Cr, Ba–Cr sulfide – 21.7 wt %–26.7 wt % Cr) (Tables 1 and S2). Like ellinaite, chromite, magnesiochromite and perovskite are also enriched in V2O3 – 7.4 wt %–8.6 wt %, 3.3 wt %–3.9 wt % and 1.5 wt %–1.9 wt %, respectively (Tables 1 and S2). In contrast to the above minerals Ca silicates and fluorapatite in the paralava are virtually free in these oxides (Table S2). It should also be mentioned that varicolored spurrite marbles at Tulul Al Hammam, central Jordan (third occurrence of ellinaite), are also characterized by high concentrations of Cr (40–1880 ppm Cr; Sokol et al., 2020), and in addition to ellinaite these rocks contain mcconnelite CuCrO2, zincochromite and magnesiochromite (Galuskina et al., 2021a, b). In general, all types of pyrometamorphic rocks of the Hatrurim Formation (Israel–Jordan) have a remarkably high concentration of Cr (Gross, 1977; Sharygin et al., 2008; Sokol et al., 2011, 2014a, b, 2019a, 2020; Geller et al., 2012; Seryotkin et al., 2019; Galuskina et al., 2021a, b).
The overview of ellinaite from the three localities assumes different PT–X–fO2 conditions for the mineral and its host rocks. The structural data for ellinaite strongly indicate the β-CaCr2O4 modification and no evidence of the precursor α-CaCr2O4. According to the phase diagram CaO-Cr2O3 (Degterov and Pelton, 1996; Shabanova et al., 2019), the phase transition between α-CaCr2O4 and β-CaCr2O4 (ellinaite) is near 1600 ∘C (Fig. S5). The mineralogy of the diamond-hosted inclusion with ellinaite from Brazil (Fig. 1) indicates lower-mantle crystallization conditions and low fO2 due to the presence of iron carbide and/or carbonitride (Kaminsky et al., 2015). Ellinaite-containing rocks of the Hatrurim Formation are characterized by near-surface pressure conditions and different T-fO2 (this work; Galuskina et al., 2021a, b). Mineral relations in the Hatrurim Basin rankinite–gehlenite paralava indicate that gehlenite, rankinite and larnite are the earliest phases crystallizing from Ca-rich silicate paralava melt (Fig. 3). Other minerals occupy interstitial spaces between the above phases and were clearly formed after them. Ellinaite crystallized after chromite but before pyrrhotite. According to experimental and calculated data for the system CaO–SiO2–Al2O3 (Mao et al., 2006; Haccuria et al., 2015), gehlenite+rankinite+larnite is the liquidus association (peritectic) at 1317–1343 ∘C, and rankinite occupies a very narrow field at temperatures near 1308 ∘C. The formation of pyrrhotite as the latest phase was probably near 1000 ∘C according to Kosyakov et al. (1996). Silicate-melt inclusions in schorlomite–rankinite–melilite paralava from the Hatrurim Basin indicate that rankinite crystallized at T>1160 ∘C (Sharygin et al., 2006).
The presence of Cr-rich pyrrhotite, wüstite, native iron, cohenite and phosphides (Table S1) in the Hatrurim rankinite–gehlenite paralava indicates highly reduced conditions. Thus, local melting of the calcareous sedimentary protolith, and crystallization of resultant rankinite–gehlenite paralava under reduced conditions with the formation of holotype ellinaite, is suggested to have occurred between 1000–1343 ∘C.
With respect to the ellinaite-bearing marbles in the Tulul Al Hammam area of Jordan, the presence of spurrite and calcite implies maximum metamorphic temperatures for these rocks of <950 ∘C (Sokol et al., 2005); Khoury et al. (2016) and Sokol et al. (2020) have subsequently determined values of 800–850 ∘C. On the other hand, Galuskina et al. (2021a, b) consider a value of ∼1000 ∘C as the maximum temperature for the ellinaite-bearing marbles and suggest the possibility that these rocks may be retrograde products of earlier pyrometamorphic crystallization with the formation of high-temperature Ca silicates and lime. The crystallization of the Tulul Al Hammam spurrite marbles with ellinaite occurred under oxidized conditions as evidenced by the presence of Fe-rich minerals (brownmillerite, srebrodolskite, hematite, andradite and priscillagrewite-(Y)), in which iron is largely present as Fe3+ (Galuskina et al., 2021a, b).
The compositional and structural data of ellinaite from three known localities show that it is stable under a wide range of PT–X–fO2 conditions, i.e., from the Earth's surface to the lower mantle. Limited solid solutions in ellinaite such as CaCrO4 (ellinaite) ↔ CaFeO4 (harmunite), CaCrO4↔ CaVO4 and CaCrO4↔ CaTiO4 involving reduced and oxidized elements reflect variable redox environments, in which ellinaite formed.
Crystallographic data for ellinaite (CIF) are available in the Supplement. This part also additionally contains four tables (Tables S1 to S4) and five figures (Figs. S1 to S5).
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-33-727-2021-supplement.
VVS provided Raman spectroscopy and SEM and microprobe analysis for the Hatrurim ellinaite and wrote the paper, with help from all coauthors. SNB performed single-crystal X-ray studies and refinement of the crystal structure for the Hatrurim ellinaite. FVK and RW conducted HRTEM and interpreted the results for the Brazilian ellinaite. ENN provided microprobe analyses and their complete processing for the Hatrurim ellinaite. GAY helped with EBSD study of the Hatrurim ellinaite. KAN identified optical properties of ellinaite. MNM provided fieldwork and collected phosphide-containing rocks at Hatrurim Basin, Israel.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Victor V. Sharygin would like to thank Mikhail V. Khlestov and Nikolai S. Karmanov (IGM, Novosibirsk, Russia) for their technical assistance during SEM studies. Special thanks for Yevgeny Vapnik (Ben-Gurion University, Israel) for providing field trips at Hatrurim Basin (Israel). The authors are grateful to the Centre for X-ray diffraction studies at the St. Petersburg State University for the access to instrumental and computational resources. We are highly appreciative of the comments, suggestions and careful editing of reviewers (Rodney Grapes, Nikita V. Chukanov).
Raman spectroscopy and EBSD investigations for the Hatrurim ellinaite were done on state assignment of IGM SB RAS (IX.125.2) and the Initiative Project of Ministry of Science and Higher Education of the Russian Federation (Act 211 of the Government of the Russian Federation (grant agreement no. 02.A03.21.0006)). SEM and microprobe studies for the Hatrurim ellinaite were supported by the Russian Science Foundation (grant no. 17-17-01056p). Crystallographic studies of the Hatrurim ellinaite were provided by the Russian Science Foundation (grant no. 18-17-00079).
This paper was edited by Cristiano Ferraris and reviewed by Rodney Grapes and Nikita V. Chukanov.
Biagioni, C. and Pasero, M.: The systematics of the spinel-type minerals: an overview, Am. Mineral., 99, 1254–1264, https://doi.org/10.2138/am.2014.4816, 2014.
Bosi, F., Biagioni, C., and Pasero, M.: Nomenclature and classification of the spinel supergroup, Eur. J. Mineral., 31, 183–192, https://doi.org/10.1127/ejm/2019/0031-2788, 2019.
Bright, N. F. H., Rowland, J. F., and Wurm, J. G.: The compound CaO.Ti2O3, Can. J. Chem., 36, 492–495, https://doi.org/10.1139/v58-070, 1958.
Britvin, S. N., Murasko, M. N., Vapnik, Y., Polekhovsky, Y. S., and Krivovichev, S. V.: Earth's phosphides in Levant and insights into the source of Archaean prebiotic phosphorus, Sci. Rep., 5, 8355, https://doi.org/10.1038/srep08355, 2015.
Britvin, S. N., Murasko, M. N., Vapnik, E., Polekhovsky, Y. S., and Krivovichev, S. V.: Barringerite Fe2P from pyrometamorphic rocks of the Hatrurim Formation, Israel, Geol. Ore Deposit, 59, 619–625, https://doi.org/10.1134/S1075701517070029, 2017.
Britvin, S. N., Murashko, M. N., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Vapnik, Y., Shilovskikh, V. V., and Lozhkin, M. S.: Nazarovite, IMA 2019-013, CNMNC Newsletter No. 50, August 2019, Mineral. Mag., 83, 615–620, https://doi.org/10.1180/mgm.2019.46, 2019a.
Britvin, S. N., Murashko, M. N., Vapnik, Y., Polekhovsky, Y. S., Krivovichev, S. V., Vereshchagin, O. S., Vlasenko, N. S., Shilovskikh, V. V., and Zaitsev, A. N.: Zuktamrurite, FeP2, a new mineral, the phosphide analogue of löllingite, FeAs2, Phys. Chem. Mineral., 46, 361–369, https://doi.org/10.1007/s00269-018-1008-4, 2019b.
Britvin, S. N., Murashko, M. N., Vereshchagin, O. S., Vapnik, Y., Shilovskikh, V. V., and Vlasenko, N. S.: Polekhovskyite, IMA 2018-147, CNMNC Newsletter No. 48, April 2019, page 401, Eur. J. Mineral., 31, 399–402, 2019c.
Britvin, S. N., Vapnik, Y., Polekhovsky, Y.S., Krivovichev, S. V., Krzhizhanovskaya, M. G., Gorelova, L. A., Vereshchagin, O. S., Shilovskikh, V. V., and Zaitsev, A. N.: Murashkoite, FeP, a new terrestrial phosphide from pyrometamorphic rocks of the Hatrurim Formation, South Levant, Mineral. Petrol., 113, 237–248, https://doi.org/10.1007/s00710-018-0647-y, 2019d.
Britvin, S. N., Murashko, M. N., Vapnik, Y., Polekhovsky, Y. S., Krivovichev, S. V., Vereshchagin, O. S., Shilovskikh, V. V., and Krzhizhanovskaya, M. G.: Negevite, the pyrite-type NiP2, a new terrestrial phosphide, Am. Mineral., 105, 422–427, https://doi.org/10.2138/am-2020-7192, 2020a.
Britvin, S. N., Murashko, M. N., Vapnik, Y., Polekhovsky, Y. S., Krivovichev, S. V., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Shilovskikh, V. V., and Vlasenko, N. S.: Transjordanite, Ni2P, a new terrestrial and meteoritic phosphide, and natural solid solutions barringerite-transjordanite (hexagonal Fe2P-Ni2P), Am. Mineral., 105, 428–436, https://doi.org/10.2138/am-2020-7275, 2020b.
Britvin, S. N., Murashko, M. N., Vapnik, Y., Polekhovsky, Y. S., Krivovichev, S. V., Vereshchagin, O. S., Shilovskikh, V. V., Vlasenko, N. S., and Krzhizhanovskaya, M. G.: Halamishite, Ni5P4, a new terrestrial phosphide in the Ni–P system, Phys. Chem. Mineral., 47, 3, https://doi.org/10.1007/s00269-019-01073-7, 2020c.
Britvin, S. N., Murashko, M. N., Vapnik, Y., Vlasenko, N. S., Krzhizhanovskaya, M. G., Vereshchagin, O. S., Bocharov, V. N., and Lozhkin, M. S.: Cyclophosphates, a new class of native phosphorus compounds, and some insights into prebiotic phosphorylation on early Earth, Geology, 49, 382–386, https://doi.org/10.1130/G48203.1, 2021a.
Britvin, S. N., Vereshchagin, O. S., Shilovskikh, V. V., Krzhizhanovskaya, M. G., Gorelova, L. A., Vlasenko, N. S., Pakhomova, A. S., Zaitsev, A. N., Zolotarev, A. A., Bykov, M., and Nestola, F.: Discovery of terrestrial allabogdanite (Fe,Ni)2P, and the effect of Ni and Mo substitution on the barringerite-allabogdanite high-pressure transition, Am. Mineral., 106, 944–952, https://doi.org/10.2138/am-2021-7621, 2021b.
Bulanova, G. P., Walter, M. J., Smith, C. B., Kohn, S. C., Armstrong, L. S., Blundy, J., and Gobbo, L.: Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juína, Brazil: subducted protoliths, carbonated melts and primary kimberlite magmatism, Contrib. Mineral. Petrol., 160, 489–510, https://doi.org/10.1007/s00410-010-0490-6, 2010.
Burg, A., Starinsky, A., Bartov, Y., and Kolodny, Y.: Geology of the Hatrurim Formation (“Mottled Zone”) in the Hatrurim basin, Israel J. Earth Sci., 40, 107–124, 1992.
Chen, M., Shu, J., Mao, H.-K., Xie, X., and Hemley, R. J.: Natural occurrence and synthesis of two new postspinel polymorphs of chromite, P. Natl. Acad. Sci. USA, 100, 14651–14654, https://doi.org/10.1073/pnas.2136599100, 2003a.
Chen, M., Shu, J., Xie, X., and Mao, H.-K.: Natural CaTi2O4-structured FeCr2O4 polymorph in the Suizhou meteorite and its significance in mantle mineralogy, Geochim. Cosmochim. Ac., 67, 3937–3942, https://doi.org/10.1016/S0016-7037(03)00175-3, 2003b.
Chen, M., Shu, J., and Mao, H.-K.: Xieite, a new mineral of high-pressure FeCr2O4 polymorph, Chinese Sci. Bull., 53, 3341–3345, 2008.
Chen, M., Shu, J., Xie, X., and Tan, D.: Maohokite, a post-spinel polymorph of MgFe2O4 in shocked gneiss from the Xiuyan crater in China, Meteor. Planet. Sci., 54, 495–502, https://doi.org/10.1111/maps.13222, 2019.
Chesnokov, B. V., Bazhenova, L. F., Bushmakin, A. F., Vilisov, A. F., Lotova, E. V., Mikhal', T. A., Nishanbaev, T. P., and Shcherbakova, E. P.: New minerals from burnt dumps of the Chelyabinsk coal basin (the 2nd report), in: New Data on the Mineralogy of Endogenic Deposits and Technogenic Zones of the Urals, edited by: Chesnokov, B. V., PH UrD AS USSR, Sverdlovsk, USSR, 5–14, 1991 (in Russian).
Damay, F., Martin, C., Hardy, V., Maignan, A., Andre, G., Knight, K., Giblin S. R., and Chapon, L. C.: Zigzag ladders with staggered magnetic chirality in the compound β-CaCr2O4, Phys. Rev. B, 81, 214405, https://doi.org/10.1103/PhysRevB.81.214405, 2010.
Degterov, S. and Pelton, A. D.: Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CrO-Cr2O3, CrO-Cr2O3-Al2O3, and CrO-Cr2O3-CaO systems, J. Phase Equilib., 17, 476–487, 1996.
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. and Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 42, 339–341, https://doi.org/10.1107/S0021889808042726, 2009.
Galuskin, E. V., Kruger, B., Kruger, H., Blass, G., Widmer, R., and Galuskina, I. O.: Wernerkrauseite, CaFeMn4+O6: the first nonstoichiometric post-spinel mineral, from Bellerberg volcano, Eifel, Germany, Eur. J. Mineral., 28, 485–493, https://doi.org/10.1127/ejm/2016/0028-2509, 2016.
Galuskina, I. O., Vapnik, Y., Lazic, B., Armbruster, T., Murashko, M., and Galuskin, E. V.: Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel, Am. Mineral., 99, 965–975, https://doi.org/10.2138/am.2014.4563, 2014.
Galuskina, I. O., Vapnik, Y., Stachowicz, M., Woźniak K., and Galuskin, E. V.: Mcconnellite, CuCrO2, and ellinaite, CaCr2O4, from varicolored spurrite marble of the Daba-Siwaqa area, Hatrurim Complex, Jordan, Min. Mag., 85, 387–397, https://doi.org/10.1180/mgm.2021.27, 2021a.
Galuskina, I., Galuskin, E., Vapnik, Y., Zeliński, G., and Prusik, K.: Priscillagrewite-(Y), (Ca2Y)Zr2Al3O12 – a new garnet of the bitikleite group from the Daba-Siwaqa area, the Hatrurim Complex, Jordan, Am. Mineral., 106, 641–649, https://doi.org/10.2138/am-2021-7692, 2021b.
Gaudefroy, C., Jouravsky, G., and Permingeat, F.: La marokite, CaMn2O4 une nouvelle espèce minérale, B. Soc. Fr. Mineral. Cr., 86, 359–367, 1963.
Geller, Y.I., Burg, A., Halicz, L., and Kolodny, Y.: System closure during the combustion metamorphic “Mottled Zone” event, Israel. Chem. Geol., 334, 25–36, https://doi.org/10.1016/j.chemgeo.2012.09.029, 2012.
Gross, S.: The mineralogy of the Hatrurim Formation, Israel. Bull. Geol. Surv. Israel, 70, 1–80, 1977.
Haccuria, E., Crivits, T., Hayes, P. C., and Jak, E.: Selected phase equilibria studies in the Al2O3-CaO-SiO2 system, J. Am. Ceram. Soc., 99, 691–704, https://doi.org/10.1111/jace.13991, 2015.
Harte, B., Harris, J. W., Hutchison, M. T., Watt, G. R., and Wilding, M. C.: Lower mantle mineral associations in diamonds from Sao Luiz, Brazil, in: Mantle petrology: field observations and high pressure experimentation, edited by: Fei, Y., Bertka, C. M., and Mysen, B. O., Geochemical Society Special Publications, Washington, USA, 125–153, 1999.
Hayman, P. C., Kopylova, M. G., and Kaminsky, F. V.: Lower mantle diamonds from Rio Soriso (Juína area, Mato Grosso, Brazil), Contrib. Mineral. Petrol., 140, 734–753, https://doi.org/10.1007/s00410-005-0657-8, 2005.
Hill, P. M., Peiser, H. S., and Rait, J. R.: The crystal structure of calcium ferrite and β calcium chromite, Acta Crystallogr., 9, 981–986, 1956.
Hörkner, W. and Müller-Buschbaum, H. K.: Einkristalluntersuchungen von β-CaCr2O4, Z. Naturforsch. B, 31, 1710–1711, 1976.
Hutchison, M. T., Hursthouse, M. B., and Light, M. E.: Mineral inclusions in diamonds: associations and chemical distinctions around the 670-km discontinuity, Contrib. Mineral. Petrol., 142, 119–126, https://doi.org/10.1007/s004100100279, 2001.
Irifune, T., Fujino, K., and Ohtani, K.: A new high pressure form of MgAl2O4, Nature, 349, 409–411, https://doi.org/10.1038/349409a0, 1991.
Kaminsky, F. V., Zakharchenko, O. D., Davies, R., Griffin, W. L., Khachatryan-Blinova, G. K., and Shiryaev, A. A.: Superdeep diamonds from the Juína area, Mato Grosso State, Brazil, Contrib. Mineral. Petrol., 140, 734–753, https://doi.org/10.1007/s004100000221, 2001.
Kaminsky, F. V., Khachatryan, G. K., Andreazza, P., Araujo, D. P., and Griffin, W. L.: Super-deep diamonds from kimberlites in the Juína area, Mato Grosso State, Brazil, Lithos, 112, 833–842, https://doi.org/10.1016/J.LITHOS.2009.03.036, 2009.
Kaminsky, F. V., Wirth, R., and Schreiber, A.: Carbonatitic inclusions in deep mantle diamond from Juína, Brazil: New minerals in the carbonate-halide association, Can. Mineral., 51, 669–688, https://doi.org/10.3749/canmin.51.5.669, 2013.
Kaminsky, F., Wirth, R., and Schreiber, A.: A microinclusion of lower mantle rock and other mineral and nitrogen lower-mantle inclusions in a diamond, Can. Mineral., 53, 83–104, https://doi.org/10.3749/canmin.1400070, 2015.
Khoury, H. N., Sokol, E. V., Kokh, S. N., Seryotkin, Y. V., Nigmatulina, E. N., Goryainov, S. V., Belogub, E. V., and Clark, I. D.: Tululite, Ca14(Fe3+,Al) (Al,Zn,Fe3+,Si,P,Mn,Mg)15O36: A new Ca zincate-aluminate from combustion metamorphic marbles, central Jordan, Mineral. Petrol., 110, 125–140, https://doi.org/10.1007/s00710-015-0413-3, 2016.
Kirby, S. H., Stein, S., Okai, E. A., and Rubie, D. C.: Metastable mantle phase transformations and deep earthquakes in subducting oceanic lithosphere, Rev. Geophys., 34, 261–306, 1996.
Kolev, N., Iliev, M. N., Popov, V. N., and Gospodinov, M.: Temperature-dependent polarized Raman spectra of CaFe2O4, Solid State Comm., 128, 153–155, https://doi.org/10.1016/S0038-1098(03)00660-4, 2003.
Kosyakov, V. I., Kraeva, A. G., Fedorova, Z. N., and Sinyakova, E. F.: Topological analysis of evolution of phase equilibria in the Fe-Ni-S system in the range XS <0.5 along the temperature axis, Geol. Geofiz., 37, 5–15, 1996.
Lee, Y. M. and Nassaralla, C. L.: Minimization of hexavalent chromium in magnesite-chrome refractory, Metall. Mater. Trans. B, 28, 855–859, 1997.
Lepicard, G. and Protas, J.: Etude structurale de l'oxyde double de manganese et decalcium orthorhombique CaMn2O4 (marokite), B. Soc. Fr. Mineral. Cr., 89, 318–324, 1966.
Ma, C. and Prakapenka, V.: Tschaunerite, IMA 2017-032a, CNMNC Newsletter No. 46, December 2018, page 1188, Eur. J. Mineral., 30, 1181–1189, 2018.
Ma, C., Tschauner, O., Beckett, J. R., Liu, Y., Greenberg, E., and Prakapenka, V. B.: Chenmingite, FeCr2O4 in the CaFe2O4-type structure, a shock-induced, high-pressure mineral in the Tissint martian meteorite, Am. Mineral., 104, 1521–1529, https://doi.org/10.2138/am-2019-6999, 2019.
Mao, H., Hillert, M., Selleby, M., and Sundman, B.: Thermodynamic assessment of the CaO-Al2O3-SiO2 system, J. Am. Ceram. Soc., 89, 298–308, https://doi.org/10.1111/j.1551-2916.2005.00698.x, 2006.
Nigmatulina, E. A.: The first finding of aciculite CaO•Fe2O3 in natural and technogenous burned rocks of the Kuznetsk Coal Basin, in: Mineralogy of Technogenesis-2006, PH IMin UrB RAS, Miass, Russia, 107–122, 2006 (in Russian).
Nigmatulina, E. N. and Nigmatulina, E. A.: Pyrogenic iron ores of fossil coal fires of the Kuznetsk coal basin, Zap. Russ. Mineral. Soc., 138, 52–68, 2009 (in Russian).
Pausch, H. and Müller Buschbaum, H. K.: Die Kristallstruktur von α-CaCr2O4, Z. Anorg. Allg. Chem., 405, 113–118, 1974.
Pouchou, I. L. and Pichoir, F.: “PaP” (phi-rho-z) procedure for improved quantitative microanalysis, in: Microbeam analysis, edited by: Armstrong, I. T., San Francisco Press, San Francisco, USA, 104–106, 1985.
Róg, G., Kozlowska-Róg, A., and Dudek, M.: The standard Gibbs free energy of formation of calcium chromium (III) oxide in the temperature range (1073 to 1273 K), J. Chem. Thermodynam., 39, 275–278, https://doi.org/10.1016/j.jct.2006.07.005, 2007.
Scheldrick, G. M.: Crystal structure refinement with SHELXL, Acta Crystallogr., C71, 3–8, https://doi.org/10.1107/S2053229614024218, 2015.
Seryotkin, Y. V., Sokol, E. V., Kokh, S. N., and Sharygin, V. V.: Natural bentorite – Cr3+-derivate of ettringite: Determination of crystal structure, Phys. Chem. Mineral., 46, 553–570, https://doi.org/10.1007/s00269-019-01022-4, 2019.
Shabanova, G. N., Korohodska, A. N., and Deviatova, N. B.: Refinement of the subsolidus structure of the four-component system Fe2O3 – CaO – Al2O3 – Cr2O3, Vopr. Khim. Khim. Tekhnol., 2, 144–149, https://doi.org/10.32434/0321-4095-2019-123-2-144-149, 2019.
Sharygin, V. V.: Mayenite-supergroup minerals from burned dump of the Chelyabinsk Coal Basin, Russ. Geol. Geophys., 56, 1603–1621, https://doi.org/10.1016/j.rgg.2015.10.007, 2015.
Sharygin, V. V.: Orthorhombic CaCr2O4 in phosphide-bearing gehlenite-rankinite paralava from Hatrurim Basin, Israel: preliminary data, in: Proceedings of 36th International Conference “Magmatism of the Earth and related strategic metal deposits”, Saint Petersburg, Russia, 23–26 May 2019, 272–276, 2019.
Sharygin, V. V., Vapnik, Y., Sokol, E. V., Kamenetsky, V. S., and Shagam, R.: Melt inclusions in minerals of schorlomite-rich veins of the Hatrurim Basin, Israel: composition and homogenization temperatures, in: ACROFI I Program with Abstracts, edited by: Ni, P. and Li, Z., Nanjing, China, 189–192, 2006.
Sharygin, V. V., Sokol, E. V., and Vapnik, Y.: Minerals of the pseudobinary perovskite–brownmillerite series from combustion metamorphic larnite rocks of the Hatrurim Formation (Israel), Russ. Geol. Geophys., 49, 709–726, https://doi.org/10.1016/j.rgg.2008.03.001, 2008.
Sharygin, V. V., Britvin, S. N., Murashko, M. N., and Vapnik, Y.: Mineralogy of phosphide-bearing gehlenite-rankinite paralavas, Hatrurim Basin, Israel, in: Proceedings of scientific conference “Mineralogical museums: yesterday, today and tomorrow”, Saint Petersburg, Russia, 17–19 September 2019, 188–190, 2019a (in Russian).
Sharygin, V. V., Yakovlev, G. A., Wirth, R., Seryotkin, Y. V., Sokol, E. V., Nigmatulina, E. N., Karmanov, N. S., and Pautov, L. A.: Nataliakulikite, Ca4Ti2(Fe3+,Fe2+)(Si,Fe3+,Al)O11, a new perovskite-supergroup mineral from Hatrurim Basin, Negev Desert, Israel, Minerals, 9, 700, https://doi.org/10.3390/min9110700, 2019b.
Sharygin, V. V., Britvin, S. N., Kaminsky, F. V., Wirth, R., Nigmatulina, E. N., Yakovlev, G. A., Novoselov, K. A., and Murashko, M. N.: Ellinaite, IMA 2019-091, in: CNMNC Newsletter 53, Eur. J. Mineral., 32, https://doi.org/10.5194/ejm-32-209-2020, 2020.
Shizuya, M., Isobe, M., and Takayama-Muromachi, E.: Structure and properties of the CaFe2O4-type cobalt oxide CaCo2O4, J. Solid State Chem., 180, 2550–2557, https://doi.org/10.1016/j.jssc.2007.07.008, 2007.
Sokol, E., Sharygin, V., Kalugin, V., Volkova, N., and Nigmatulina, E.: Fayalite and kirschsteinite solid solutions in melts from burned spoil-heaps, South Urals, Russia, Eur. J. Mineral., 14, 795–807, https://doi.org/10.1127/0935-1221/2002/0014-0795, 2002.
Sokol, E., Novikov, I., Zateeva, S., Vapnik, Y., Shagam, R., and Kozmenko, O.: Combustion metamorphic rocks as indicators of fossil mud volcanism: New implications for the origin of the Mottled Zone, Dead Sea area, Basin Res., 22, 414–438, https://doi.org/10.1111/j.1365-2117.2010.00462.x, 2010.
Sokol, E. V., Maksimova, N. V., Nigmatulina, E. N., Sharygin, V. V., and Kalugin, V. M.: Combustion Metamorphism, PH SB RAS, Novosibirsk, 284 pp., ISBN 5-7692-0783-3, 2005 (in Russian).
Sokol, E. V., Novikov, I. S., Zateeva, S. N., Sharygin, V. V., and Vapnik, Y.: Pyrometamorphic rocks of the spurrite-merwinite facies as indicators of hydrocarbon discharge zones (the Hatrurim Formation, Israel), Dokl. Earth Sci., 420, 608–614, https://doi.org/10.1134/S1028334X08040181, 2008.
Sokol, E. V., Gaskova, O. L., Kokh, S. N., Kozmenko, O. A., Seryotkin, Y. V., Vapnik, Y., and Murashko, M. N.: Chromatite and its Cr3+- and Cr6+-bearing precursor minerals from the Nabi Musa Mottled Zone complex, Judean Desert, Am. Mineral., 96, 659–674, https://doi.org/10.2138/am.2011.3487, 2011.
Sokol, E. V., Kozmenko, O. A., Kokh, S. N., and Vapnik, Y.: Gas reservoirs in the Dead Sea area: Evidence from chemistry of combustion metamorphic rocks in Nabi Musa fossil mud volcano, Russ. Geol. Geophys., 53, 745–762, https://doi.org/10.1016/j.rgg.2012.06.003, 2012.
Sokol, E. V., Gaskova, O. L., Kozmenko, O. A., Kokh, S. N., Vapnik, E. A., Novikova, S. A., and Nigmatulina, E. N.: Clastic dikes of the Hatrurim basin (western flank of the Dead Sea) as natural analogues of alkaline concretes: Mineralogy, solution chemistry, and durability, Dokl. Earth Sci., 459, 1436–1441, https://doi.org/10.1134/S1028334X14100122, 2014a.
Sokol, E. V., Kokh, S. N., Vapnik, Y., Thiery, V., and Korzhova, S. A.: Natural analogs of belite sulfoaluminate cement clinkers from Negev Desert, Israel, Am. Mineral., 99, 1471–1487, https://doi.org/10.2138/am.2014.4704, 2014b.
Sokol, E. V., Seryotkin, Y. V., Kokh, S. N., Vapnik, Y., Nigmatulina, E. N.; Goryainov, S. V., Belogub, E. V., and Sharygin, V. V.: Flamite (Ca,Na,K)2(Si,P)O4, a new mineral from the ultrahigh-temperature combustion metamorphic rocks, Hatrurim Basin, Negev Desert, Israel, Mineral. Mag., 79, 583–596, https://doi.org/10.1180/minmag.2015.079.3.05, 2015.
Sokol, E. V., Kokh, S. N., Khoury, H. N., Seryotkin, Y. V., Goryainov, S. V., Novikova, S. A., and Sokol, I. A.: Natural analogue approaches to prediction of long-term behaviour of Ca2UO5•2-3H2O X-phase: Case study from Tulul Al Hammam site, Jordan, Arab. J. Geosci., 10, 512, https://doi.org/10.1007/s12517-017-3305-5, 2017.
Sokol, E. V., Kokh, S. N., Sharygin, V. V., Danilovsky, V. A., Seryotkin, Y. V., Liferovich, R., Deviatiiarova, A. S., Nigmatulina, E. N., and Karmanov, N. S.: Mineralogical diversity of Ca2SiO4-bearing combustion metamorphic rocks in the Hatrurim Basin: Implications for storage and partitioning of elements in oil shale clinkering, Minerals, 9, 465, https://doi.org/10.3390/min9080465, 2019a.
Sokol, E. V., Polyansky, O. P., Semenov, A. N., Reverdatto, V. V., Kokh, S. N., Devyatiyarova, A. S., Kolobov, V. Y., Khvorov, P. V., and Babichev, A. V.: High-grade contact metamorphism in the Kochumdek River valley (Podkamennaya Tunguska basin, East Siberia): Evidence for magma flow, Russ. Geol. Geophys., 60, 386–399, https://doi.org/10.15372/GiG2019088, 2019b.
Sokol, E. V., Kokh, S. N., Seryotkin, Y. V., Deviatiiarova, A. S., Goryainov, S. V., Sharygin, V. V., Khoury, H. N., Karmanov, N. S., Danilovsky, V. A., and Artemyev D. A.: Ultrahigh-temperature sphalerite from Zn-Cd-Se-rich combustion metamorphic marbles, Daba Complex, Central Jordan: paragenesis, chemistry, and structure, Minerals, 10, 822, https://doi.org/10.3390/min10090822, 2020.
Stoe & Cie: X-AREA (Version 1.35) and X-RED32 (Version 1.31), Stoe & Cie GmbH, Darmstadt, Germany, 2006.
Toth, S., Lake, B., Kimber, S. A. J., Pieper, O., Reehuis, M., Islam, A. T. M. N., Zaharko, O., Ritter, C., Hill, A. H., Ryll, H., Kiefer, K., Argyriou, D. N., and Williams, A. J.: 120∘ helical magnetic order in the distorted triangular antiferromagnet α-CaCr2O4, Phys. Rev. B, 84, 054452, https://doi.org/10.1103/PhysRevB.84.054452, 2011.
Vapnik, Y., Sharygin, V., Sokol, E., and Shagam, R.: Paralavas in a combustion metamorphic complex, Hatrurim Basin, Israel, GSA Rev. Engin. Geol., 18, 133–154, https://doi.org/10.1130/2007.4118(09), 2007.
Walter, M., Bulanova, G., Armstrong, L., Keshav, S., Blundy, J. D., Gudfinnsson, G., Lord, O., Lennie, A., Smith, C., and Gobbo, L.: Primary carbonatite melt from deeply subducted oceanic crust, Nature, 454, 622–625, https://doi.org/10.1038/nature07132, 2008.
Zateeva, S. N., Sokol, E. V., and Sharygin, V. V.: Specificity of pyrometamorphic minerals of the ellestadite group, Geol. Ore Deposit, 49, 792–805, https://doi.org/10.1134/S1075701507080132, 2007.
Xue, W., Zhai, K., and Zhai, S.: Thermal expansion of ellinaite (β-CaCr2O4): an in-situ high temperature X-ray diffraction study, Phys. Chem. Mineral., 48, 2, https://doi.org/10.1007/s00269-020-01126-2, 2021.
Zhai, S., Yin, Y., Shieh, S. R. Shan, S., Xue, W., Wang, C.-P., Yang, K., and Higo, Y.: High-pressure X-ray diffraction and Raman spectroscopy of CaFe2O4-type β-CaCr2O4, Phys. Chem. Mineral., 43, 307–314, https://doi.org/10.1007/s00269-015-0795-0, 2016.
- Abstract
- Introduction
- General data for ellinaite from Brazil
- Analytical methods for the Hatrurim Basin ellinaite
- General information for ellinaite-bearing paralava at Hatrurim Basin, Israel
- Morphology and optical and physical properties of ellinaite from Hatrurim Basin
- Chemical composition of the holotype specimen (Hatrurim Basin)
- Structural data for ellinaite from Hatrurim Basin
- Discussion and final remarks
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- General data for ellinaite from Brazil
- Analytical methods for the Hatrurim Basin ellinaite
- General information for ellinaite-bearing paralava at Hatrurim Basin, Israel
- Morphology and optical and physical properties of ellinaite from Hatrurim Basin
- Chemical composition of the holotype specimen (Hatrurim Basin)
- Structural data for ellinaite from Hatrurim Basin
- Discussion and final remarks
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement