Changes to the cerite group nomenclature
The cerite and merrillite groups belong to the cerite supergroup. Some nomenclature and classification changes have been made to the cerite group, whereas the merrillite group remains unchanged. Minerals of the cerite group have the general formula A9XM[T7O24Ø4]Z3, where T is Si. The cerite group, from now on, is subdivided into two subgroups, cerite and taipingite. The root name will be cerite and taipingite if the Z anions are dominated by (OH) and F, respectively. The prefix ferri- or alumino- will be added if the M cations are dominated by Fe3+ or Al, respectively. If the M cation is Mg, there will be no prefix. Taking into account the valency-imposed double site occupancy and the site total charge approach, a double suffix will be used to represent the essential A constituents in the general chemical formula. Cerite-(Ce), aluminocerite-(Ce), ferricerite-(La), and taipingite-(Ce) have been renamed cerite-(CeCa), aluminocerite-(CeCa), ferricerite-(LaCa), and taipingite-(CeCa), respectively. The newly approved mineral aluminotaipingite-(CeCa) also belongs to the taipingite subgroup.
Table 1Ideal formula, dominant constituents in the general chemical formula, and unit-cell parameters (R3c) for the cerite supergroup minerals.
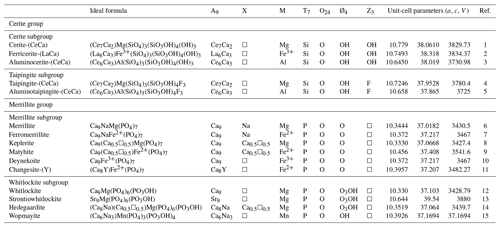
1 Moore and Shen (1983). 2 Pakhomovsky et al. (2002). 3 Nestola et al. (2009). 4 Qu et al. (2020). 5 Campostrini et al. (2023). 6 Xie et al. (2015). 7 Britvin et al. (2016). 8 Britvin et al. (2021). 9 Hwang et al. (2019). 10 Galuskin et al. (2023). 11 Li et al. (2022). 12 Calvo and Gopal (1975). 13 Britvin et al. (1991). 14 Witzke et al. (2015). 15 Cooper et al. (2013).
The cerite supergroup (Atencio and Azzi, 2020) consists of two groups of isostructural trigonal R3c (no. 161) minerals, namely, cerite (silicates) and merrillite (phosphates) groups. The merrillite group is subdivided into two subgroups: merrillite (without OH in the Ø site) and whitlockite (with OH in the Ø site). The nomenclature introduced by Atencio and Azzi (2020) is based on the dominant species of the dominant valence at each site.
The recent discoveries of four new members in the cerite group, following the four valid members already approved, make it necessary to revise the nomenclature of this group. One of these new species, aluminotaipingite-(CeCa) IMA2022-126, has already been approved by the CNMNC-IMA (Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association) and published (Campostrini et al., 2023); two are under review by the CNMNC-IMA; and one is under study.
The crystal structure of these minerals involves three 8- and 9-fold coordinated A sites, one 6-fold coordinated X site, one octahedral M site, and three [TO3(Ø)] tetrahedral groups. The crystal structure of cerite supergroup minerals consists of [M(TO4)6] clusters linked by {A9X(TO3Ø)} groups.
The general chemical formula of cerite supergroup minerals is A9XM[T7O24Ø4]Z3, where the letters, except for O for oxygen, represent groups of atoms (and not structural sites) on which to apply the dominant constituent and valency rule and the endmember concept. A = REE, Ca, Sr, Na, and □; X = □, Ca, Na, and Fe2+; M = Mg, Fe2+, Fe3+, Al, and Mn; T = Si and P; Ø = O and OH; and Z = □, OH, and F, where REE is rare earth elements of yttrium and lanthanoids (La–Lu) (Atencio and Azzi, 2020). The general structural formula of cerite supergroup minerals is A13A23A33XM1[T13T23T3O24Ø13Ø10]Z1Z2Z3. The letter Z represents the set of anions occurring at three nonequivalent Z1, Z2, and Z3 sites; thus, the composition Z = F1.8(OH)1.2 is consistent with the endmember composition Z = F3 (as F > OH). Grouping atoms over similar sites, such as A(1,2,3), T(1,2,3), and Z(1,2,3), helps prevent the proliferation of mineral species with endmember formulae based on the structural formula, where each structural sites can be used (Nickel and Grice, 1998) to define an endmember formula (Hawthorne, 2002). For example, the three independent anion Z sites may result in an endmember composition like F2(OH). In this way, cerite nomenclature is based on the chemical formula, and, in principle, only the chemical information is needed for the mineral identification.
The cerite group, from now on, is subdivided into two subgroups, cerite and taipingite.
The root name will be cerite and taipingite if the Z anions are dominated by (OH) and F, respectively. The prefix ferri- or alumino- will be added if the M cations are dominated by Fe3+ or Al, respectively. If the M cation is Mg, there will be no prefix. Taking into account the valency-imposed double site occupancy (Hatert and Burke, 2008; Hatert et al., 2013) and the site total charge approach of Bosi et al. (2019), a double suffix will be used to represent the essential A constituents in the general chemical formula. Cerite-(Ce), aluminocerite-(Ce), ferricerite-(La), and taipingite-(Ce) have been renamed cerite-(CeCa), aluminocerite-(CeCa), ferricerite-(LaCa), and taipingite-(CeCa), respectively. The newly approved mineral aluminotaipingite-(CeCa) also belongs to the taipingite subgroup.
The charge balance of cerite group minerals is according to the following substitution mechanism: A[REE8□]+24 + M(Fe,Al)3+ = A[REE6(Ca,Sr)3]+24 +M(Fe,Al)[REE7(Ca,Sr)2]+25 + M(Mg,Fe)2+.
The nomenclature of merrillite group remains as it current is. Table 1 summarizes all the currently valid species in the cerite supergroup.



