the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Freitalite, C14H10, a new aromatic hydrocarbon mineral from Freital, Saxony, Germany
Thomas Witzke
Martin Schreyer
Benjamin Brandes
René Csuk
Herbert Pöllmann
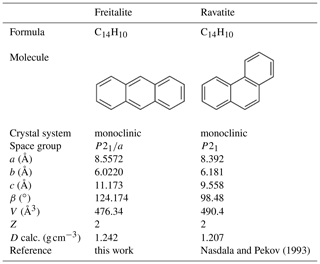
The new mineral species freitalite, C14H10, corresponding to the aromatic hydrocarbon anthracene, has been discovered on the mine dump of the Königin Carola shaft (also named Paul Berndt Mine), Freital, near Dresden, Saxony, Germany. The mineral forms thin blades or flakes of irregular shape up to a few millimetres in size and shows an intense violet or whitish-violet to white colour. Freitalite is a product of pyrolysis of coal at low oxygen fugacity and was formed by sublimation from a gas phase. The mineral is associated with sulfur and hoelite. Elemental analysis gave (in wt. %, average of three analyses) C 94.07, H 5.571 and total 99.641. The empirical formula is C14.00H9.88 (calculated for C = 14). The identity with anthracene was confirmed by infrared and Raman spectroscopy, high-performance liquid chromatography, gas chromatography with mass spectrometry, 1H and 13C NMR spectrometry, and X-ray powder diffraction. Freitalite is monoclinic, P21∕a, with lattice parameters a=8.5572(9), b=6.0220(5), c=11.173(1) Å, β=124.174(1)∘ and V=476.34(3) Å3 refined from powder data. The calculated density of 1.242 g cm−3 (for Z=2) is very close to the measured density of 1.240 g cm−3. Freitalite was accepted as a new mineral by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA 2019-116).
- Article
(2649 KB) - Full-text XML
-
Supplement
(188 KB) - BibTeX
- EndNote
The mineral was found in 1987 and 1988 on the mine dump of the coal mine Königin Carola shaft (Paul Berndt Mine), Freital, Döhlen basin, near Dresden, Saxony, Germany, by one of the authors (Thomas Witzke). Further studies showed that it corresponds to the organic compound anthracene, C14H10 (Witzke, 1990).
The aromatic hydrocarbon compound C14H10 is known with two isomers: anthracene and phenanthrene. The latter was described as the mineral ravatite (IMA 1992-019) from a burning coal seam near Ravat, Tajikistan (Nasdala and Pekov, 1993). The submission of anthracene as a new mineral was prevented for decades because of its formation on a burning mine dump.
Since the beginning of the 1990s, phases formed on burning mine dumps were not accepted as minerals anymore (Ernest H. Nickel, personal communication, 1991). The formal decision of the International Mineralogical Association (IMA) Commission on New Minerals and Mineral Names that “as a general rule, products of combustion are not to be considered minerals in the future” was published by Nickel and Grice (1998). One of the reasons given was that the possibility of human intervention in originating a fire could not be excluded.
According to the new guideline of the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the IMA (Parafiniuk and Hatert, 2020), phases forming on burning coal dumps with no human agency initiating the fire and no anthropogenic material deposited there should be treated as minerals. The mine dump of the Königin Carola shaft meets these criteria; there is no indication of human activities in initiating the fire or anthropogenic material deposited there. The new mineral and the name freitalite were accepted by the CNMNC in March 2020 (IMA 2019-116).
The co-type specimens of freitalite are deposited in the Mineralogical Collection of the TU Bergakademie Freiberg, Saxony, Germany, no. MiSa72396 (used for XRD, Raman, IR, HPLC, MS) and MiSa84590 (used for XRD, Rietveld analysis, density, elemental analysis, 1H NMR, 13C NMR).
The mineral kratochvilite, C13H10 (fluorene), is very similar in visual appearance and chemical composition. It is originally described from the burning dumps of the mines Max and Schoeller at Libušin near Kladno, Czech Republic (Rost, 1937). The type samples of kratochvilite in the National Museum, Prague, were lost during the German occupation end of the 1930s. Material from an additional sample in the museum, no. 30345, labelled as kratochvilite by Rost, could also be used for the study.
The type locality of freitalite is the mine dump of the Königin Carola shaft, Freital, near Dresden, Saxony, Germany (51.00277∘ N, 13.63827∘ E). The coal mine was active from 1872 to 1959. In 1948, the mine was renamed to Paul Berndt Mine, but the original name, usually in the short form Carola shaft, stayed very popular. Around 1960 the mine dump caught fire by the well-known process of spontaneous self-ignition. Self-heating and spontaneous combustion processes of coal and the influence of parameters like the iron sulfide content, coal lithology, and water and oxygen supply are described in numerous papers, e.g. Nelson and Chen (2007), Onifade and Genc (2018), Sýkorová et al. (2018), Stracher et al. (2010, 2015), and references therein. As a result of the combustion processes, a complex suite of gases like CO2, SO2, SO3, NH3, HCl, H2S, H2Se, different hydrocarbons and several other compounds can be emitted and lead to the formation of minerals by condensation, sublimation, chemical reaction of gases, leaching of rocks by acidic solutions and metasomatic reactions (Witzke et al., 2015; Sýkorová et al., 2018).
The mine dump of the Königin Carola shaft consists of natural rocks surrounding the coal (shale, sandstone, conglomerate) and residual coal. The coal is generally rich in sulfidic sulfur, which promotes the spontaneous self-ignition. The sulfur content in the common coal was in the range of 2 %–5 % but could reach in some lithotypes up to 30 % (27 % sulfidic as pyrite). Very pyrite-rich coal shows strong oxidation effects already after a few days exposure to air (Reichel, 1984). The mine dump was renovated and recultivated in 2014 and the fire is extinguished.
Freitalite is associated with sulfur and rarely with hoelite (anthraquinone, C14H8O2). The crystals are usually grown on sulfur and sometimes overgrown by sulfur. The minerals formed as a result of the combustion processes on the mine dump were described by Witzke (1990). The other C14H10 isomer ravatite (phenanthrene) was found very rarely on the mine dump but not directly associated with freitalite (Witzke, 1995). Freitalite is a product of pyrolysis of the coal at low oxygen fugacity and was formed by sublimation from a gas phase. The mineral was found in fumaroles 5 to 40 cm below the surface in a temperature range of 20–70 ∘C. Some of the data obtained for freitalite were already published (Witzke, 1990; Thalheim et al., 1991; Nasdala et al., 1993). The formation of aromatic hydrocarbons as pyrolysis products and deposition at lower temperatures near the surface of burning dumps, including anthracene, is described by Akulov and Akulova (2020).
The mineral was also identified on a sample labelled “kratochvilite” from a burning coal mine dump at Libušin near Kladno, Bohemia, Czech Republic. Small violet blades from the burning mine dump of the Becker shaft, Hänichen near Dresden, Saxony, Germany, named “Anilinviolett” by Groth (1867), might be, according to the chemical tests and the given short description, identical with freitalite.
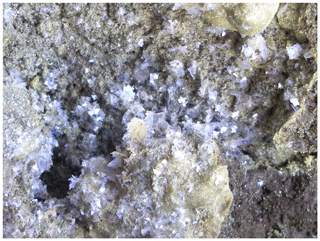
Freitalite forms thin blades or flakes of irregular shape up to a few millimetres in size, rarely up to nearly 1 cm, with the dominant form {001} (Fig. 1). The larger flakes are usually composed of several crystals, intergrown parallel (001) in different orientations. The thickness of small blades (single crystals) is around 1 µm. The blades are often curved. The mineral shows an intense violet or whitish-violet to white colour. The streak is white, the lustre vitreous to pearly and the Mohs hardness is 1. The blades are flexible and malleable. Thin crystals are transparent and larger flakes translucent. Freitalite shows an intense bluish-white fluorescence in short- and long-wave ultraviolet light (254 and 366 nm). The density of 1.240 g cm−3 (average of two measurements, 1.238 and 1.242 g cm−3) was determined by flotation in sodium polytungstate solution.
For the natural material, only a refractive index α=1.57 (white light) could be measured. Single crystals were too thin to measure β. Larger blades have variable thickness and are composed of crystals in different orientations. The value for γ is outside the range of suitable refractive liquids. The freitalite crystals were slowly attacked by the refractive liquids. In transmitted light the mineral is colourless; X=Z without pleochroism. For synthetic material, parameters α=1.56, β=1.80, γ=2.19, 2V (meas.) =87∘, 2V (calc.) =89∘, Y=b, ∘ were found (Obreimov et al., 1948; Colombo and Mathieu, 1960).
Chemical data for freitalite were obtained by high-performance liquid chromatography (HPLC), gas chromatography (GC) with mass spectrometry (MS) and CHN elemental analysis.
HPLC was performed using LiChrosorb RP-8 columns and as reference materials synthetic anthracene (C14H10), synthetic phenanthrene (C14H10), synthetic fluorene (C13H10) and synthetic anthraquinone (C14H8O2). One of the co-type samples of freitalite from Freital, Germany, gave 98.3 % anthracene, 0.4 % phenanthrene, 0.1 % fluorene and 1.2 % not identified (possibly dianthracene). A sample labelled kratochvilite from Libušin near Kladno, Czech Republic, gave a very similar result: 97.1 % anthracene, 0.6 % phenanthrene and 1.8 % not identified (possibly dianthracene) and is therefore also freitalite. Sample no. 30345 from the National Museum, Prague, Czech Republic, labelled kratochvilite from Libušin near Kladno, was found by HPLC analysis to be a mixture of anthracene and phenanthrene in a ratio close to 1:1 with anthracene slightly dominant and only traces of fluorene (Witzke, 1990).
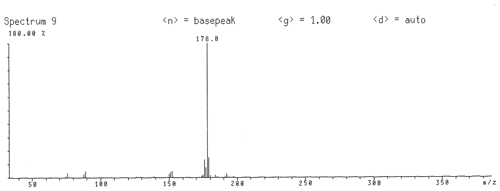
Figure 2Gas chromatography with mass spectrometry data of freitalite from Freital. The spectrum corresponds completely to the spectrum of synthetic anthracene (NIST Chemistry WebBook, 2018a).
GC/MS investigation (electron ionization) of a material from a co-type sample of freitalite (Fig. 2) gave a spectrum corresponding completely to the spectrum of synthetic anthracene (NIST Chemistry WebBook, 2018a).
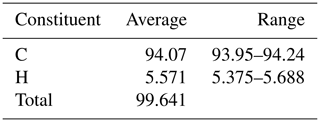
For the elemental analysis, a Foss Heraeus Vario EL (C-HNS) instrument was used. The average of three analyses and the measured range are given in Table 1. From the analyses, an empirical formula of C14.00H9.88 based on C = 14 could be calculated. This is very close to the ideal composition of anthracene, C14H10, which requires C 94.34, H 5.66 and total 100 wt. %.
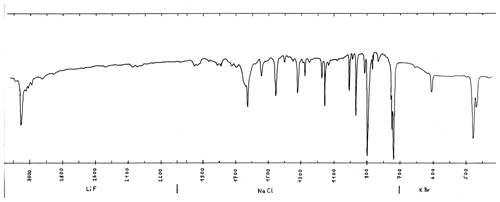
The infrared spectrum (measured in transmission mode, with KBr, NaCl and LiF prisms) was measured on material from a co-type sample from Freital, Germany (Witzke, 1990). The spectrum (Fig. 3) corresponds completely to the one from anthracene (NIST Chemistry WebBook, 2018b), except the shoulder at 1650 cm−1, which may be from a slight contamination. The spectrum can be easily distinguished from the spectrum of the isomer phenanthrene.
The Raman spectrum of a sample from Freital, measured with a GDM-1000 instrument (135∘ configuration, excitation line Ar+ 5154 Å, 0.07 mW), corresponds to anthracene and can be easily distinguished from the spectrum of the isomer phenanthrene. The data were already published (Nasdala et al., 1993).
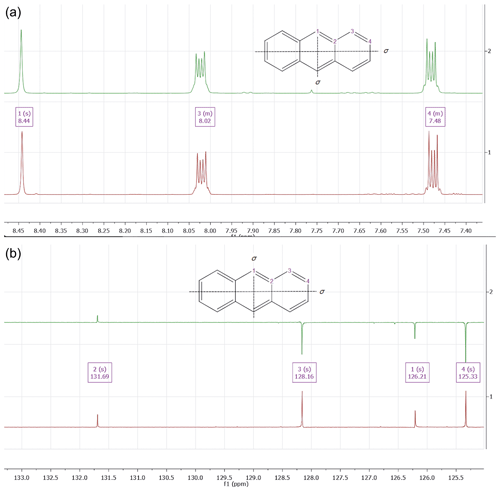
Figure 41H (a) and 13C NMR spectrum (b) of a sample of freitalite (green colored) as well as an authentic sample of anthracene (red colored). The NMR spectra were recorded using the Varian spectrometers Gemini 2000 or Unity 500 in CDCl3; residual un-deuterated solvent used for referencing, 20 ∘C.
The 1H NMR spectrum in CDCl3 (Fig. 4) showed three major signals being centered at δ=8.44 ppm (broad singlet, assigned to two hydrogens 1-H), δ=8.02 ppm (multiplet, assigned to four hydrogens 3-H) and a multiplet at δ=7.48 ppm (assigned to four hydrogens 4-H). The position of the signals, their multiplicity and the integrals correspond well with the signals observed for an authentic sample (anthracene, ReagentPlus, 99 %, Merck, 141062) and data from the literature (Sharpless et al., 1974). The presence of small amounts of impurities was visible in the spectrum.
The 13C NMR spectrum in CDCl3 spectrum of freitalite is characterized by the presence of four signals at δ=131.7 ppm (assigned to the four carbons C-2), δ=128.2 ppm (assigned to four carbons C-3), δ=126.2 (assigned to two carbons C-1) and a signal at δ=125.3 ppm (assigned to four carbons C-4). The position of the signals corresponds very well with the observed signal positions of an authentic sample. Their assignment was based on spectra simulation (MestReNova calculation, vers. 14.01) as well as literature data (Caspar et al., 1975). The sample was not completely pure, as can be seen from the observed impurities.
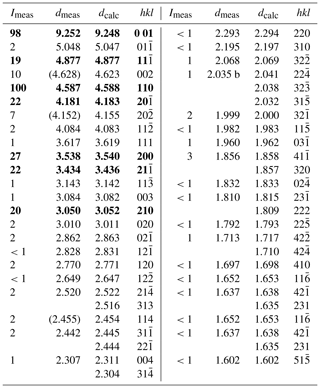
X-ray powder diffraction data of freitalite were collected with an Empyrean diffractometer (Malvern Panalytical) using Cu Kα radiation. The instrument was equipped with a focusing mirror, 0.02 rad Soller slits and PIXcel3D detector. The powdered sample was measured in a 0.5 mm capillary. From the powder data (Table 2), the lattice parameter for a monoclinic cell with a=8.5572(9), b=6.0220(5), c=11.173(1) Å, β=124.174(1)∘ and V=476.34(3) Å3 were refined by a Pawley fit in the HighScore Plus 4.8 software (Malvern Panalytical). The calculated density of 1.242 g cm−3 based on the empirical formula and the refined cell volume for Z=2 is very close to the measured value.
The crystal structure of anthracene with the linear and planar arrangement of the benzene rings in the molecules was first determined by Robertson (1933). He found a monoclinic cell, P21∕a, with a=8.58, b=6.02, c=11.18 Å, β=125∘ and Z=2. Redeterminations and refinements were done by Mathieson et al. (1950), Sinclair et al. (1950), Cruickshank (1956) and Mason (1964). Studies at different temperatures and pressures were done by Ponomarev and Shilov (1983), Brock and Dunitz (1990), and Oehzelt et al. (2003). All the studies confirmed principally the data obtained by Robertson (1933). The unit cell is usually given as described by Robertson (1933) for better comparison with phenanthrene. Sometimes the cell is given in the standard setting in space group P21∕c, a=9.463, b=6.026, c=8.550 Å, β=103.57∘, V=473.94 Å3, Z=2 (Wang, 1978), with the same cell volume as the one described in P21∕a.
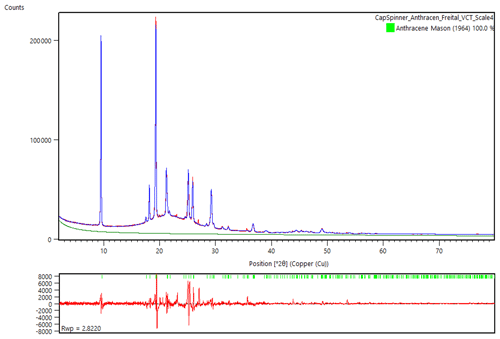
Figure 5Rietveld refinement of the powder diffraction data of freitalite, using the structure data from Mason (1964). Atomic positions or occupation parameters were not refined. The hump in the scan is from the glass capillary. Glass contribution was modelled from an empty capillary.
A Rietveld refinement (Fig. 5) was performed using the powder diffraction data from the measurement in the capillary and the program HighScore Plus 4.8 (Malvern Panalytical). For the refinement, the data of the structure determination of anthracene by Mason (1964) were used, converted to the standard setting P21∕c. Atomic positions or occupation parameters were not refined. The contribution of the glass was modelled from the measurement of an empty capillary. In the Rietveld refinement of freitalite (R.exp. 1.375, R Profile 2.126, Weighted R Profile 2.822, Goodness of Fit 2.053, R Bragg 0.46), the lattice parameters a=9.516, b=6.018, c=8.557 Å, β=103.883∘ and V=475.76 Å3 were obtained, confirming the identification as anthracene.
The crystallographic data of the two C14H10 isomers freitalite and ravatite are compared in Table 3.
XRD and Rietveld data are available in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/ejm-33-1-2021-supplement.
TW found the mineral, made XRD analyses and Rietveld refinements, determined physical and optical properties, obtained spectroscopic data, and wrote the paper. MS made XRD analyses and Rietveld refinements. BB and RC made the CHN, 1H and 13C NMR analyses. HP provided additional data.
The authors declare that they have no conflict of interest.
The authors acknowledge Jaroslav Švenek (†), National Museum, Prague, Czech Republic, for providing material from a sample of kratochvilite (no. 30345) in 1989.
This paper was edited by Francisca Martinez-Ruiz and reviewed by Jan Jehlicka, Cristobal Verdugo-Escamilla and one anonymous referee.
Akulov, N. I. and Akulova, V. V.: Pyrolysis of technogenic re-deposited coal-bearing rocks of spoil heaps, Geosciences, 10, 122–134, https://doi.org/10.3390/geosciences10040122, 2020.
Brock, C. P. and Dunitz, J. D.: Temperature dependence of thermal motion in crystalline anthracene, Acta Cryst., B46, 795–806, 1990.
Caspar, M. L., Stothers, J. B., and Wilson, N. K.: 13C Nuclear magnetic resonance studies of methylated anthracenes, Can. J. Chem., 53, 1958–1969, 1975.
Colombo, L. and Mathieu, J.-P.: Propriétés optiques et spectres de diffusion des cristaux d'anthracène, Bull. Soc. franç. Min. Crist., 83, 250–254, 1960.
Cruickshank, D. W. J.: A detailed refinement of the crystal and molecular structure of anthracene, Acta Cryst., 9, 915–923, 1956.
Groth, P.: Ueber neugebildete Brandproducte auf einem brennenden Steinkohlenfelde bei Dresden, Sitzungsberichte Isis-Gesellschaft Dresden, Jg. 1867, 68–70, 1867.
Mason, R.: The crystallography of anthracene at 95 ∘K and 290 ∘K, Acta Cryst., 17, 547–555, 1964.
Mathieson, Mc. L., Robertson, J. M., and Sinclair, V. C.: The crystal and molecular structure of anthracene. I. X-ray measurements, Acta Cryst., 3, 245–250, 1950.
Nasdala, L. and Pekov, I. V.: Ravatite, C14H10, a new organic mineral species from Ravat, Tadzhikistan, Eur. J. Mineral., 5, 699–705, 1993.
Nasdala, L., Pekov, I. V., and Witzke, T.: Raman investigation of naturally occurring C14H10, Chemie der Erde, 53, 59–69, 1993.
Nelson, M. I. and Chen, X. D.: Survey of experimental work on the self-heating and spontaneous combustion of coal, in:Geology of Coal Fires: Case Studies from Around the World: Geological Society of America Reviews in Engineering Geology, edited by: Stracher, G. B., XVIII, 31–83, 2007.
Nickel, E. H. and Grice, J. E.: The IMA Commission on New Minerals and Mineral Names: Procedure and guidelines on mineral nomenclature, 1998, Miner. Petrol., 64, 237–263, 1998.
NIST Chemistry Webbook, Anthracene: GC-MS: available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C120127&Units=SI&Mask=200#Mass-Spec (last access: 7 January 2021), 2018a.
NIST Chemistry Webbook, Anthracene: Infra-red: available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C120127&Type=IR-SPEC&Index=1 (last access: 7 January 2021), 2018b.
Obreimov, I. V., Prikhot'ko, A. F., and Rodnikova, I. V.: Dispersion of anthracene in the visible part of the spectrum, Zhurnal Experimental'noi i Theoreticheskoi Fisikii, 18, 409, 1948 (in Russian).
Oehzelt, M., Heimel, G., Resel, R., Puschnig, P., Hummer, K., Ambrosch-Draxl, C., Takemura, K., and Nakayama, A.: High pressure X-ray study on anthracene, J. Chem. Phys., 119, 1078–1084, 2003.
Onifade, M. and Genc, B.: Spontaneous combustion of coal and coal shales, Int. J. Mining Sci. Technol., 28, 933–940, 2018.
Parafiniuk, J. and Hatert, F.: New IMA CNMNC guidelines on combustion products from burning coal dumps, Eur. J. Mineral., 32, 215–217, https://doi.org/10.5194/ejm-32-215-2020, 2020.
Ponomarev, V. I. and Shilov, G. V.: Crystal structure of anthracene in the 300–100 K range, Kristallografiya, 28, 674–677, 1983 (in Russian).
Reichel, W.: Die Kohlelithotypen und ihre Bildungsräume in den Steinkohlenflözen des Döhlener Beckens bei Dresden, Hercynia N.F., 21, 319–334, 1984.
Robertson, J. M.: The crystalline structure of anthracene. A quantitative X-ray investigation, P. Roy. Soc. A, 140, 79–98, 1933.
Rost, R.: Minerály hořících hald na Kladensku. Rozpravy Česke Akad. 47, 11, 1-19, 1937 (in Czech).
Sharpless, N. E., Bradley, R. B., and Ferretti, J. A.: The nuclear magnetic resonance of heterocyclic compounds related to anthracene, Organ. Magn. Resonan., 6, 115–120, 1974.
Sinclair, V. C., Robertson, J. M., and Mathieson, Mc. L.: The crystal and molecular structure of anthracene. II. Structure investigation by the Triple Fourier Series Method, Acta Cryst., 3, 251–256, 1950.
Stracher, G. B., Prakash, A., and Sokol, E. V. (Eds.): Coal and Peat Fires. A Global Perspective. Volume 1. Coal – Geology and Combustion, Elsevier, Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo, 380 p., 2010.
Stracher, G. B., Prakash, A., and Sokol, E. V. (Eds.): Coal and Peat Fires. A Global Perspective. Volume 3. Case Studies – Coal Fires, Elsevier, Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo, 816 p., 2015.
Sýkorová, I., Kříbek, B., Havelcová, M., Machoviča, V., Laufek, F., Veselovský, F., Špaldoňová, A., Lapčák, L., Knésl, I., Matysová, P., and Majer, V.: Hydrocarbon condensates and argillites in the Eliška Mine burnt coal waste heap of the Žacléř coal district (Czech Republic): Products of high- and low temperature stages of self-ignition, Int. J. Coal Geol., 190, 146–165, 2018.
Thalheim, K., Reichel, W., and Witzke, T.: Die Minerale des Döhlener Beckens, Schriften des Staatlichen Museums für Mineralogie und Geologie zu Dresden, No. 3, 130 p., 1991.
Wang, P.: Anthracene. ICDD Grant in aid, No. 00-030-1525, JCPDS International Centre for Diffraction Data, Newtown Square, PDF 4, 1978.
Witzke, T.: Sekundärminerale und Haldenbrandminerale des Döhlener Beckens, Diploma thesis, Bergakademie Freiberg, Sektion Mineralogie/Geochemie, 77 p. + attachments, 1990.
Witzke, T.: Neufunde aus Sachsen (IV): Neue Nachweise der seltenen Minerale Chernikovit, Ktenasit, Letovicit, Ramsbeckit, Ravatit und Znucalit, Lapis, 20, 35–36, 1995.
Witzke, T., de Wit, F., Kolitsch, U., and Blass, G.: Mineralogy of the burning Anna I coal mine dump, in: Coal and Peat Fires. A Global Perspective. Volume 3. Case Studies – Coal Fires, edited by: Stracher, G. B., Prakash, A., and Sokol, E. V., Elsevier, Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo, p. 203–240, 2015.